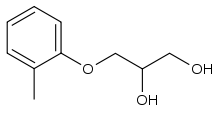
Mephenesin
Mephenesin is a centrally acting muscle relaxant. It can be used as an antidote for strychnine poisoning. Mephenesin however presents with the major drawbacks of having a short duration of action and a much greater effect on the spinal cord than the brain, resulting in pronounced respiratory depression at clinical doses and therefore a very low therapeutic index. It is especially dangerous and potentially fatal in combination with alcohol and other depressants.[1] Mephenesin was used by Dr. Bernard Ludwig and Dr. Frank Milan Berger to synthesize meprobamate, the first tranquilizer to see widespread clinical use. Mephenesin is no longer available in North America but is used in France, Italy and a few other countries.[2] Its use has largely been replaced by the related drug methocarbamol, which is better absorbed[3]
See also
External links
- Bachmeyer C, Blum L, Fléchet M, Duriez P, Cabane J, Imbert J (1996). "[Severe contact dermatitis caused by mephenesin]". Ann Dermatol Venereol 123 (3): 185–7. PMID 8761781.
- Ono H, Nakamura T, Ito H, Oka J, Fukuda H (1987). "Rigidity in rats due to radio frequency decerebration and effects of chlorpromazine and mephenesin". Gen Pharmacol 18 (1): 57–9. doi:10.1016/0306-3623(87)90170-4. PMID 3557053.
References
|
|---|
| Peripherally acting
(primarily antinicotinic,
NMJ block) | Non-depolarizing | |
|---|
| Depolarizing | |
|---|
| | |
|---|
|
|---|
| | Centrally acting | | |
|---|
| Benzodiazepines | |
|---|
| Nonbenzodiazepines | |
|---|
| Thienodiazepines | |
|---|
| Quinazolines | |
|---|
| Anticholinergics
(Antimuscarinics) | |
|---|
| Other | |
|---|
|
|---|
| | Directly acting | |
|---|
| |
|---|
| | Description |
- Anatomy
- head
- neck
- arms
- chest and back
- diaphragm
- abdomen
- genital area
- legs
- Muscle tissue
- Physiology
|
|---|
| | Disease |
- Myopathy
- Soft tissue
- Connective tissue
- Congenital
- abdomen
- muscular dystrophy
- Neoplasms and cancer
- Injury
- Symptoms and signs
|
|---|
| | Treatment |
- Procedures
- Drugs
- anti-inflammatory
- muscle relaxants
|
|---|
|
|