Medical use of nitroglycerin
 | |
.png) | |
| Systematic (IUPAC) name | |
|---|---|
|
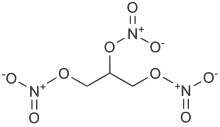
1,3-dinitrooxypropan-2-yl nitrate; [3-(nitrooxy)-2-[(nitrooxy)methyl]propyl] nitrate | |
| Clinical data | |
| AHFS/Drugs.com | nitroglycerin |
| MedlinePlus | a601086 |
| |
| |
| Sublingual, transdermal, oral, intravenous | |
| Pharmacokinetic data | |
| Bioavailability | <1% |
| Metabolism | Hepatic (rapid), Red blood cells, vascular wall |
| Half-life | 3 minutes |
| Excretion | In urine, in bile |
| Identifiers | |
|
55-63-0 | |
| C01DA02 C05AE01 | |
| PubChem | CID 4510 |
| DrugBank |
DB00727 |
| ChemSpider |
4354 |
| UNII |
G59M7S0WS3 |
| ChEBI |
CHEBI:28787 |
| ChEMBL |
CHEMBL730 |
| Chemical data | |
| Formula | C3H5N3O9 |
| 227.087 g/mol | |
|
SMILES
| |
| |
| | |
The medical use of nitroglycerin is for the treatment of angina and heart failure. The chemical nitroglycerin is commonly referred to as "glyceryl trinitrate" or "GTN" in medicine to separate it from nitroglycerin used for explosions.
Its use began with the experiments of William Murrell, which were reported in 1879. It is on the WHO Model List of Essential Medicines, a list of the most important medication needed in a basic health system.[1] The mechanism of nitric oxide (NO) generation from GTN and the metabolic consequences of this bioactivation are not entirely understood.
Medical uses
Nitroglycerin is used for the treatment of angina,[2] acute myocardial infarction,[2] severe hypertension, and coronary artery spasms .
It is useful in decreasing angina attacks, perhaps more so than reversing angina once started, by supplementing blood concentrations of nitric oxide, also called endothelium-derived relaxing factor, before the structure of the nitric oxide as the responsible agent was known. This led to the development of transdermal patches of glyceryl trinitrate, providing 24-hour release.[3] However, the effectiveness of glyceryl trinitrate is limited by development of tolerance/tachyphylaxis within 2–3 weeks of sustained use. Continuous administration and absorption (such as provided by daily pills and especially skin patches) accelerate onset of tolerance and limit the usefulness of the agent. Thus glyceryl trinitrate works best when used only short term, pulse dosing. Glyceryl trinitrate is useful for acute myocardial infarction (heart attack) and pulmonary edema, again working best if used quickly, within a few minutes of symptom onset, as a pulse dose. It may also be given as a sublingual or buccal dose in the form of a tablet placed under the tongue or a spray into the mouth for the treatment of an angina attack.[2]
Glyceryl trinitrate is also used in the treatment of anal fissures, though usually at a much lower concentration than that used for angina treatment.[4]
Adverse effects
After long-term use for chronic conditions, tolerance may develop in a patient, reducing its effectiveness. Nitrate tolerance was first described soon after the introduction of GTN in cardiovascular therapy as the loss of symptomatic and hemodynamic effects of GTN and/or the need for higher doses of the drug to achieve the same effects. The mechanisms of nitrate tolerance have been thoroughly investigated in the last 30 years and several hypotheses have been proposed. These include:
- Impaired biotransformation of GTN to its active principle - NO (or a NO-related species)
- Neurohormonal activation, causing sympathetic activation and release of vasoconstrictors such as endothelin and angiotensin II, which counteract the vasodilation induced by GTN
- Plasma volume expansion
- The oxidative stress hypothesis (proposed by Munzel et al. in 1995).
Recent evidence suggests that the latter hypothesis might represent a unifying hypothesis, and a GTN-induced inappropriate production of oxygen free radicals might induce a number of abnormalities which include those described above. Furthermore, studies have shown that nitrate tolerance is associated with vascular abnormalities which have the potential to worsen patients' prognosis (Nakamura et al.). These include endothelial and autonomic dysfunction (Gori et al.).
In the short run, glyceryl trinitrate can cause severe headaches, necessitating analgesic (very rarely up to morphine) administration for relief of pain, severe hypotension, and, in certain cases, bradycardia. This makes some physicians nervous and should prompt caution when starting nitrate administration. The painful nature of these adverse effects has a marked negative impact on patient compliance.
GTN used together with vasodilators that combat erectile dysfunction, such as Viagra, Cialis, or Levitra can cause severe hypotension, circulatory collapse, and death.
Dangers
It is often recommended that GTN transdermal patches should be removed before defibrillation[5][6] due to the risk of explosion, but careful investigations have concluded that reports of apparent GTN patch explosions during defibrillation are due to voltage breakdown involving the metal mesh in some patches.[7]
Mechanism of action
GTN is a prodrug which must first be denitrated to produce the active metabolite nitric oxide (NO). Nitrates which undergo denitration within the body to produce NO are called nitrovasodilators and their denitration occurs via a variety of mechanisms. The mechanism by which nitrates produce NO is widely disputed. Some believe that nitrates produce NO by reacting with sulfhydryl groups, while others believe that enzymes such as glutathione S-transferases, cytochrome P450 (CYP), and xanthine oxidoreductase are the primary source of GTN bioactivation. In recent years a great deal of evidence has been produced that supports the belief that clinically relevant denitration of GTN to produce 1,2-glyceryl dinitrate (GDN) and NO is catalysed by mitochondrial aldehyde dehydrogenase (mtALDH). NO is a potent activator of guanylyl cyclase (GC) by heme-dependent mechanisms; this activation results in cGMP formation from cyclic guanosine triphosphate (cGTP). Thus, NO increases the level of cGMP within the cell. cGMP then activates myosin light chain phosphatase via a cGMP-dependent protein kinase.
History
It was known almost from the time of its discovery by Ascanio Sobrero in 1846-7 that the tasting or close handling of nitroglycerin could cause sudden intense headaches, which indicated some form of vasodilation effect. This was reported by Sobrero. Constantine Hering developed a sublinguial dose form of nitroglycerin in 1847 and advocated for it as a treatment of a number of diseases. However, it was not known to be a specific treatment for blood pressure and chest pain.
Following Thomas Brunton's discovery that amyl nitrite could be used to treat chest pain, William Murrell experimented with the use of nitroglycerin to alleviate angina pectoris and reduce blood pressure, and proved that the headaches occurred due to overdose. He began treating patients with small doses in 1878, and the substance was soon adopted into widespread use after he published his results in The Lancet in 1879. The medical establishment used the name "glyceryl trinitrate" or "trinitrin" to avoid alarming patients, who knew that nitroglycerin was explosive.[8]
Research
A 2007 medical development includes a small amount of nitroglycerin (trademark as Zanifil Gel) in the tip of a new Durex condom to stimulate erection during intercourse. The manufacturer says "The CSD500 condom contains a chemical in its teat, called glyceryl trinitrate (GTN), which is absorbed by the skin and causes blood vessels to dilate." Jokes were made about dynamite, and the company standing proud.[9][10]
Notes
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ 2.0 2.1 2.2 JRCALC National Clinical Guidelines GTN http://www2.warwick.ac.uk/fac/med/research/hsri/emergencycare/prehospitalcare/jrcalcstakeholderwebsite/guidelines/glyceryl_trinitrate_gtn_suscard_gtn.pdf
- ↑ medicines.org. "mknitro" (PDF). Retrieved 16 September 2011.
- ↑ "Medicinenet info on Anal Fissures". Medicinenet.com. Retrieved 2009-01-21.
- ↑ http://www.resus.org.uk/pages/als.pdf
- ↑ http://www.ser.nl/nl/grenswaarden/~/media/Files/Internet/Grenswaarden/SCOEL/GSW524_SCOEL_Prov147Nitroglycerine.ashx
- ↑ Liddle, R.; Richmond, W. (1998). "Investigation into voltage breakdown in glyceryl trinitrate patches". Resuscitation 37 (3): 145–148. doi:10.1016/S0300-9572(98)00059-8. PMID 9715773.
- ↑ Sneader, Walter. Drug Discovery: A History, p433. John Wiley and Sons, 2005. ISBN 0-471-89980-1
- ↑ Futura Medical
- ↑ CNN Money
References
- Marsh N, Marsh A. (2000). "A short history of nitroglycerine and nitric oxide in pharmacology and physiology". Clin Exp Pharmacol Physiol 27 (4): 313–9. doi:10.1046/j.1440-1681.2000.03240.x. PMID 10779131.
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||