Median arcuate ligament syndrome
| Median arcuate ligament syndrome | |
|---|---|
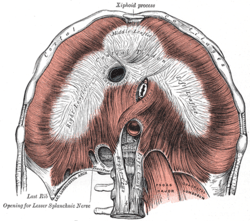 Median arcuate ligament syndrome results from compression of the celiac artery by the median arcuate ligament. The median arcuate ligament is a fibrous arch formed by the left and right diaphragmatic crura, visible here on the underside of the diaphragm. | |
| Classification and external resources | |
| ICD-10 | I77.4 |
| ICD-9 | 447.4 |
| eMedicine | article/188618 |
In medicine, the median arcuate ligament syndrome (MALS, also known as celiac artery compression syndrome, celiac axis syndrome, celiac trunk compression syndrome or Dunbar syndrome) is a condition characterized by abdominal pain attributed to compression of the celiac artery and possibly the celiac ganglia by the median arcuate ligament.[1] The abdominal pain may be related to meals, may be accompanied by weight loss, and may be associated with an abdominal bruit heard by a clinician. It is also called celiac artery compression syndrome.
The diagnosis of MALS is one of exclusion, as many healthy patients demonstrate some degree of celiac artery compression in the absence of symptoms. Consequently, a diagnosis of MALS is typically only entertained after more common conditions have been ruled out. Once suspected, screening for MALS can be done with ultrasonography and confirmed with computed tomography (CT) or magnetic resonance (MR) angiography.
Treatment is generally surgical, the mainstay being open division, or separation, of the median arcuate ligament combined with removal of the celiac ganglia. The majority of patients benefit from surgical intervention. Poorer responses to treatment tend to occur in patients of younger age, those with a psychiatric condition or who use alcohol, have abdominal pain unrelated to meals, or who have not experienced weight loss.
Anatomy and pathogenesis
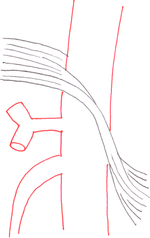

The median arcuate ligament is a ligament formed at the base of the diaphragm where the left and right diaphragmatic crura join near the 12th thoracic vertebra. This fibrous arch forms the anterior aspect of the aortic hiatus, through which the aorta, thoracic duct, and azygos vein pass. The median arcuate ligament usually comes into contact with the aorta above the branch point of the celiac artery. However, in up to one quarter of normal individuals, the median arcuate ligament passes in front of the celiac artery, compressing the celiac artery and nearby structures such as the celiac ganglia.[1] In some of these individuals, this compression is pathologic and leads to the median arcuate ligament syndrome.[1]
Several theories attempt to explain the origin of pain caused by compression of the celiac artery.[2] One proposes that compression of the celiac artery causes ischemia, or decreased blood flow, to abdominal organs, leading to pain. Another hypothesizes that there is compression not only of the celiac artery but also of the celiac ganglia, and that pain results from compression of the latter.
Epidemiology
It is estimated that in 10-24% of normal, asymptomatic individuals the median arcuate ligament crosses in front of (anterior to) the celiac artery, causing some degree of compression.[1][3] Approximately 1% of these individuals exhibit severe compression associated with symptoms of MALS.[1] The syndrome most commonly affects individuals between 20 and 40 years old, and is more common in women, particularly thin women.[1]
Signs and symptoms
Patients with MALS reportedly experience abdominal pain, particularly in the epigastrium, which may be associated with eating and which may result in anorexia and weight loss.[1] Other signs are persistent nausea, lassitude (especially after a heavy meal) and exercise intolerance. Exercise or certain postures can aggravate the symptoms. Occasionally, physical examination reveals an abdominal bruit in the mid-epigastrium.[1]
Complications of MALS result from chronic compression of the celiac artery. They include gastroparesis[4] and aneurysm of the pancreaticoduodenal arteries.[5]
Diagnosis
Median arcuate ligament syndrome is a diagnosis of exclusion.[1][2] That is, the diagnosis of MALS is generally considered only after patients have undergone an extensive evaluation of their gastrointestinal tract including upper endoscopy, colonoscopy, and evaluation for gallbladder disease and gastroesophageal reflux disease (GERD).[2]
The diagnosis of MALS relies on a combination of clinical features and findings on medical imaging.[1] Clinical features include those signs and symptoms mentioned above; classically, MALS involves a triad of abdominal pain after eating, weight loss, and an abdominal bruit, although the classic triad is found in only a minority of individuals that carry a MALS diagnosis.[2]
Diagnostic imaging for MALS is divided into screening and confirmatory tests.[2] A reasonable screening test for patients with suspected MALS is duplex ultrasonography to measure blood flow through the celiac artery.[2][6] Peak systolic velocities greater than 200 cm/s are suggestive of celiac artery stenosis associated with MALS.[2]
|
Further evaluation and confirmation can be obtained via angiography to investigate the anatomy of the celiac artery.[2] Historically, conventional angiography was used, although this has been largely replaced by less invasive techniques such as computed tomography (CT) and magnetic resonance (MR) angiography.[1][2] Because it provides better visualization of intraabdominal structures, CT angiography is preferred to MR angiography in this setting.[2] The findings of focal narrowing of the proximal celiac artery with poststenotic dilatation, indentation on the superior aspect of the celiac artery, and a hook-shaped contour of the celiac artery support a diagnosis of MALS.[1] These imaging features are exaggerated on expiration, even in normal asymptomatic individuals without the syndrome.[1]
Proximal celiac artery stenosis with poststenotic dilatation can be seen in other conditions affecting the celiac artery.[1] The hook-shaped contour of the celiac artery is characteristic of the anatomy in MALS and helps distinguish it from other causes of celiac artery stenosis such as atherosclerosis.[1] This hooked contour is not entirely specific for MALS however, given that 10-24% of normal asymptomatic individuals have this anatomy.[1]
Treatment
Decompression of the celiac artery is the general approach to treatment of MALS.[2] The mainstay of treatment involves an open surgical approach to divide, or separate, the median arcuate ligament to relieve the compression of the celiac artery.[2] This is combined with removal of the celiac ganglia and evaluation of blood flow through the celiac artery, for example by intraoperative duplex ultrasound. If blood flow is poor, celiac artery revascularization is usually attempted; methods of revascularization include aortoceliac bypass, patch angioplasty, and others.[2]
A laparoscopic approach may also be used to achieve celiac artery decompression;[7] however, should the celiac artery require revascularization, the procedure would require conversion to an open approach.[2]
Endovascular methods such as percutaneous transluminal angioplasty (PTA) have been used in patients who have failed open and/or laparoscopic intervention.[2] PTA alone, without decompression of the celiac artery, may not be of benefit.[2][8]
Prognosis
There are few studies of the long-term outcomes of patients treated for MALS.[2] According to Duncan,[2] the largest and more relevant late outcomes data come from a study of 51 patients who underwent open surgical treatment for MALS, 44 of whom were available for long-term follow-up at an average of nine years following therapy.[9] The investigators reported that among patients who underwent celiac artery decompression and revascularization, 75% remained asymptomatic at follow-up. In this study, predictors of favorable outcome included:
- Age from 40 to 60 years
- Lack of psychiatric condition or alcohol use
- Abdominal pain that was worse after meals
- Weight loss greater than 20 lb (9.1 kg)
History
Celiac artery compression was first observed by Benjamin Lipshutz in 1917.[10] MALS was first described by Pekka-Tapani Harjola in 1963[11] and subsequently by J. David Dunbar and Samuel Marable in 1965.[12] It has also been called Harjola-Marable syndrome and Marable syndrome.[10]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 1.10 1.11 1.12 1.13 1.14 1.15 1.16 Horton KM, Talamini MA, Fishman EK (2005). "Median arcuate ligament syndrome: evaluation with CT angiography". Radiographics 25 (5): 1177–82. doi:10.1148/rg.255055001. PMID 16160104.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 Duncan AA (April 2008). "Median arcuate ligament syndrome". Curr Treat Options Cardiovasc Med 10 (2): 112–6. doi:10.1007/s11936-008-0012-2. PMID 18325313.
- ↑ Lindner HH, Kemprud E (November 1971). "A clinicoanatomical study of the arcuate ligament of the diaphragm". Arch Surg 103 (5): 600–5. doi:10.1001/archsurg.1971.01350110102016. PMID 5117015.
- ↑ Balaban DH, Chen J, Lin Z, Tribble CG, McCallum RW (March 1997). "Median arcuate ligament syndrome: a possible cause of idiopathic gastroparesis". Am. J. Gastroenterol. 92 (3): 519–23. PMID 9068484.
- ↑ Manghat NE, Mitchell G, Hay CS, Wells IP (September 2008). "The median arcuate ligament syndrome revisited by CT angiography and the use of ECG gating--a single centre case series and literature review". Br J Radiol 81 (969): 735–42. doi:10.1259/bjr/43571095. PMID 18541631.
- ↑ Sproat IA, Pozniak MA, Kennell TW (November 1993). "US case of the day. Median arcuate ligament syndrome (celiac artery compression syndrome)". Radiographics 13 (6): 1400–2. doi:10.1148/radiographics.13.6.8290734. PMID 8290734.
- ↑ Carbonell AM, Kercher KW, Heniford BT, Matthews BD (May 2005). "Multimedia article. Laparoscopic management of median arcuate ligament syndrome". Surg Endosc 19 (5): 729. doi:10.1007/s00464-004-6010-x. PMID 15965588.
- ↑ Matsumoto AH, Tegtmeyer CJ, Fitzcharles EK et al. (1995). "Percutaneous transluminal angioplasty of visceral arterial stenoses: results and long-term clinical follow-up". J Vasc Interv Radiol 6 (2): 165–74. doi:10.1016/S1051-0443(95)71087-9. PMID 7787348.
- ↑ Reilly LM, Ammar AD, Stoney RJ, Ehrenfeld WK (January 1985). "Late results following operative repair for celiac artery compression syndrome". J. Vasc. Surg. 2 (1): 79–91. doi:10.1067/mva.1985.avs0020079. PMID 3965762.
- ↑ 10.0 10.1 synd/4106 at Who Named It?
- ↑ Harjola PT (1963). "A rare obstruction of the coeliac artery. Report of a case". Ann Chir Gynaecol Fenn 52: 547–50. PMID 14083857.
- ↑ Dunbar JD, Molnar W, Beman FF, Marable SA (November 1965). "Compression of the celiac trunk and abdominal angina". Am J Roentgenol Radium Ther Nucl Med 95 (3): 731–44. PMID 5844938.