Maxwell construction

In thermodynamic equilibrium, a necessary condition for stability is that pressure 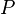 does not increase with volume
does not increase with volume 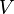 . This basic consistency requirement—and similar ones for other conjugate pairs of variables—are sometimes violated in analytic models for first order phase transitions. The most famous case is the Van der Waals equation for real gases, see Fig. 1 where a typical isotherm is drawn (black curve). The Maxwell construction is a way of correcting this deficiency. The decreasing right hand part of the curve in Fig. 1 describes a diluted gas, while its left part describes a liquid. The intermediate (rising) part of the curve in Fig. 1 would be correct, if these two parts were to be joined smoothly—meaning in particular that the system would remain also in this region spatially uniform with a well defined density. But this is not what happens. If the volume of a vessel containing a fixed amount of liquid is expanded at constant temperature, there comes a point where some of the liquid boils and the system consists of two well separated phases. While this two-phase coexistence holds as the volume continues to increase, the pressure remains constant. It decreases again, after all liquid is evaporated and the gas expands. Thus the sinusoidal part of the isotherm is replaced by a horizontal line (red line in Fig. 1). According to the Maxwell construction (or "equal area rule"), the height of the horizontal line is such that the two green areas in Fig. 1 are equal. The Maxwell construction is derived from the condition that the Gibbs free energies of the gas and the liquid must be equal when they coexist. Essentially the same applies to any other thermodynamic system, where
. This basic consistency requirement—and similar ones for other conjugate pairs of variables—are sometimes violated in analytic models for first order phase transitions. The most famous case is the Van der Waals equation for real gases, see Fig. 1 where a typical isotherm is drawn (black curve). The Maxwell construction is a way of correcting this deficiency. The decreasing right hand part of the curve in Fig. 1 describes a diluted gas, while its left part describes a liquid. The intermediate (rising) part of the curve in Fig. 1 would be correct, if these two parts were to be joined smoothly—meaning in particular that the system would remain also in this region spatially uniform with a well defined density. But this is not what happens. If the volume of a vessel containing a fixed amount of liquid is expanded at constant temperature, there comes a point where some of the liquid boils and the system consists of two well separated phases. While this two-phase coexistence holds as the volume continues to increase, the pressure remains constant. It decreases again, after all liquid is evaporated and the gas expands. Thus the sinusoidal part of the isotherm is replaced by a horizontal line (red line in Fig. 1). According to the Maxwell construction (or "equal area rule"), the height of the horizontal line is such that the two green areas in Fig. 1 are equal. The Maxwell construction is derived from the condition that the Gibbs free energies of the gas and the liquid must be equal when they coexist. Essentially the same applies to any other thermodynamic system, where 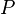 and
and 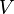 are replaced by a different pair of conjugate variables, e.g. magnetic field and magnetization or
chemical potential and number of particles.
are replaced by a different pair of conjugate variables, e.g. magnetic field and magnetization or
chemical potential and number of particles.
See also
References
- Maxwell, J. C. (1875). "On the Dynamical Evidence of the Molecular Constitution of Bodies.". Nature 11: 357–359. Bibcode:1875Natur..11..357C. doi:10.1038/011357a0.
- Reichl, L. E. (2009). A Modern Course in Statistical Physics (3rd ed.). New York, NY USA: Wiley-VCH. ISBN 9783527407828.