Malate synthase
| malate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.3.3.9 | ||||||||
| CAS number | 9013-48-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction
- acetyl-CoA + H2O + glyoxylate
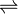 (S)-malate + CoA
(S)-malate + CoA
The 3 substrates of this enzyme are acetyl-CoA, H2O, and glyoxylate, whereas its two products are (S)-malate and CoA.
This enzyme belongs to the family of transferases, specifically those acyltransferases that convert acyl groups into alkyl groups on transfer. The systematic name of this enzyme class is acetyl-CoA:glyoxylate C-acetyltransferase (thioester-hydrolysing, carboxymethyl-forming). Other names in common use include L-malate glyoxylate-lyase (CoA-acetylating), glyoxylate transacetylase, glyoxylate transacetase, glyoxylic transacetase, malate condensing enzyme, malate synthetase, malic synthetase, and malic-condensing enzyme. This enzyme participates in pyruvate metabolism and glyoxylate and dicarboxylate metabolism.
Structure and Isoforms
Malate synthases fall into two major families, isoforms A and G. Isoform G is monomeric with a size of ~80-kD and found exclusively in bacteria.[1] Isoform A is about ~65 kD per subunit, and can form homomultimers in eukaryotes.[2] This enzyme contains a central TIM barrel sandwiched between an N-terminal alpha-helical clasp and an alpha/beta domain stemming from two insertions into the TIM barrel sequence. Then the enzyme ends with a C-terminal five-helix plug. The active site, where the acetyl-CoA and glyoxylate bind to the enzyme, lie between the TIM barrel and C-terminal plug.[3] Upon binding, the acetyl-CoA molecule forms a J-shape inserted into the binding pocket, by an intramolecular hydrogen bond between N7 of the adenine ring and a hydroxyl group on the pantetheine tail.[3] In addition, a critical magnesium ion within the active site coordinates with glyoxylate, glutamic acid 427, aspartic acid 455, and two water molecules.[3] The amino acids interacting with acetyl CoA upon binding are highly conserved.[1] Sequence identity is high within each class of isoforms, but between both classes sequence identity drops to about 15%.[4] The alpha/beta domain, which has no apparent function, is not seen in isoform A.[5]
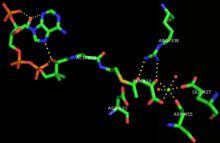
Mechanism
The mechanism of malate synthase is a combination of a Claisen condensation and a hydrolysis of an acyl-CoA. Initially, the aspartic acid 631 acts as a catalytic base, abstracting a proton from the alpha carbon of acetyl-CoA and creating a enolate that is stabilized by Arginine 338.[5] This is considered to be the rate-determining step of the mechanism.[6] Then the newly-formed enolate acts as a nucleophile that attacks the aldehyde of the glyoxylate molecule, imparting a negative charge on the oxygen which is stabilized by the Arginine 338 and the coordinating magnesium cation. This malyl-CoA intermediate then undergoes hydrolysis at the acyl-CoA portion, replacing it with an carboxylate anion.[1] The enzyme is free to release the malate and the coenzyme A molecules.
Function
Malate synthase works together with isocitrate lyase in the glyoxylate cycle to bypass two oxidative steps of Krebs cycle and permit carbon incorporation from acetate or fatty acids in many microorganisms.[7] As a result, the cell would not need to lose 2 molecules of carbon dioxide when entering the glyoxylate cycle rather than the Krebs cycle. This pathway is especially important to M. tuberculosis, allowing long-term persistence of its infection.[1] When the M. tuberculosis becomes phagocytosed, the bacterium upregulates genes encoding the glyoxylate shunt enzymes.[8] Since this cycle is not found in humans and other mammals, malate synthase is perceived as a future drug target against tuberculosis and other microorganisms. Within germinating plants, the glyoxylate cycle allows the conversion of reserve lipids into carbohydrates within glyoxysomes.[9]
Structural studies
As of late 2007, 4 structures have been solved for this class of enzymes, with PDB accession codes 1P7T, 1Y8B, 2GQ3, and 2JQX.
References
- ↑ 1.0 1.1 1.2 1.3 Smith, C. V.; Huang, C. C.; Miczak, A; Russell, D. G.; Sacchettini, J. C.; Höner Zu Bentrup, K (2003). "Biochemical and structural studies of malate synthase from Mycobacterium tuberculosis". Journal of Biological Chemistry 278 (3): 1735–43. doi:10.1074/jbc.M209248200. PMID 12393860.
- ↑ Durchschlag, H; Biedermann, G; Eggerer, H (1981). "Large-scale purification and some properties of malate synthase from baker's yeast". European journal of biochemistry / FEBS 114 (2): 255–62. PMID 7011808.
- ↑ 3.0 3.1 3.2 Anstrom, D. M.; Kallio, K.; Remington, S. J. (2003). "Structure of the Escherichia colimalate synthase G:pyruvate:acetyl-coenzyme a abortive ternary complex at 1.95 Å resolution". Protein Science 12 (9): 1822. doi:10.1110/ps.03174303.
- ↑ Serrano, J. A.; Bonete, M. J. (2001). "Sequencing, phylogenetic and transcriptional analysis of the glyoxylate bypass operon (ace) in the halophilic archaeon Haloferax volcanii". Biochimica et biophysica acta 1520 (2): 154–62. PMID 11513957.
- ↑ 5.0 5.1 Howard, B. R.; Endrizzi, J. A.; Remington, S. J. (2000). "Crystal structure of Escherichia coli malate synthase G complexed with magnesium and glyoxylate at 2.0 a resolution: Mechanistic implications". Biochemistry 39 (11): 3156–68. PMID 10715138.
- ↑ Clark, J. D.; O'Keefe, S. J.; Knowles, J. R. (1988). "Malate synthase: Proof of a stepwise Claisen condensation using the double-isotope fractionation test". Biochemistry 27 (16): 5961–71. PMID 2847778.
- ↑ Kornberg, H. L.; Sadler, J. R. (1961). "The metabolism of C2-compounds in micro-organisms. VIII. A dicarboxylic acid cycle as a route for the oxidation of glycollate by Escherichia coli". The Biochemical journal 81: 503–13. PMC 1243371. PMID 14458448.
- ↑ Höner Zu Bentrup, K; Miczak, A; Swenson, D. L.; Russell, D. G. (1999). "Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis". Journal of bacteriology 181 (23): 7161–7. PMC 103675. PMID 10963599.
- ↑ Smith, C. V.; Huang, C. C.; Miczak, A; Russell, D. G.; Sacchettini, J. C.; Höner Zu Bentrup, K (2003). "Biochemical and structural studies of malate synthase from Mycobacterium tuberculosis". Journal of Biological Chemistry 278 (3): 1735–43. doi:10.1074/jbc.M209248200. PMID 12393860.
- DIXON GH, KORNBERG HL, LUND P (1960). "Purification and properties of malate synthetase". Biochim. Biophys. Acta. 41 (2): 217–33. doi:10.1016/0006-3002(60)90004-4. PMID 13816984.
| |||||||||||||||||||||