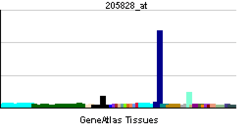
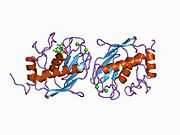
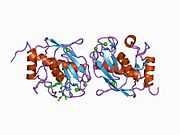
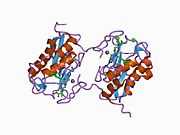
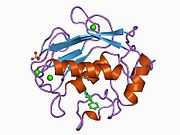
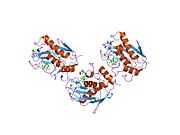
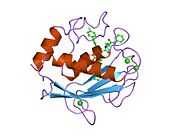
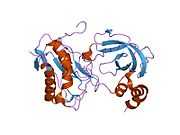
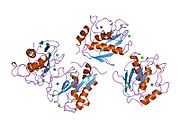
MMP3
Stromelysin-1 also known as matrix metalloproteinase-3 (MMP-3) is an enzyme that in humans is encoded by the MMP3 gene. The MMP3 gene is part of a cluster of MMP genes which localize to chromosome 11q22.3.[1] MMP-3 has an estimated molecular weight of 54 kDa.[2]
Function
Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix and during tissue remodeling in normal physiological processes, such as embryonic development and reproduction, as well as in disease processes, such as arthritis, and tumour metastasis. Most MMPs are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. The MMP-3 enzyme degrades collagen types II, III, IV, IX, and X, proteoglycans, fibronectin, laminin, and elastin. In addition, MMP-3 can also activate other MMPs such as MMP-1, MMP-7, and MMP-9, rendering MMP-3 crucial in connective tissue remodeling.[3] The enzyme is thought to be involved in wound repair, progression of atherosclerosis, and tumor initiation. Nuclear MMP3 functions as a transcription factor and a proteinase.[4]
Gene regulation
Expression of MMP3 is primarily regulated at the level of transcription, where the promoter of the gene responds to various stimuli, including growth factors, cytokines, tumor promoters, and oncogene products.[5] A polymorphism in the promoter of the MMP3 gene was first reported in 1995.[6] The polymorphism is due to variation in the number of adenosines located at position -1171 relative to the transcription start site, resulting in one allele having five adenosines (5A) and the other allele having six adenosines (6A). In vitro promoter functional analyses showed that the 5A allele had greater promoter activities as compared with the 6A allele.[3] It has been shown in different studies that individuals carrying the 5A allele have increased susceptibility to diseases attributed to increased MMP expression, such as acute myocardial infarction and abdominal aortic aneurysm.[7][8] On the other hand, the 6A allele has been found to be associated with diseases characterized by insufficient MMP-3 expression due to a lower promoter activity of the 6A allele, such as progressive coronary atherosclerosis.[3][9][10] The -1171 5A/6A variant has also been associated with congenital anomalies such as cleft lip and palate, where individuals with cleft lip/palate presented significantly more 6A/6A genotypes than controls.[11] MMP3 as a transcription factor regulates CTGF/CCN2 gene. [4]
Recently, the MMP3 gene was shown to be down-regulated in individuals with cleft lip and palate when compared to controls,[12] reinforcing the nature of cleft lip/palate as a condition resulting from insufficient or defective embryonic tissue remodeling.
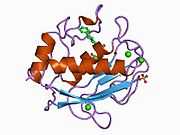
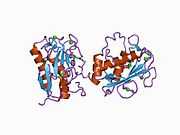
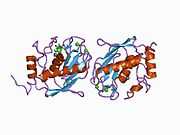
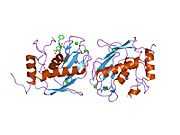
Structure
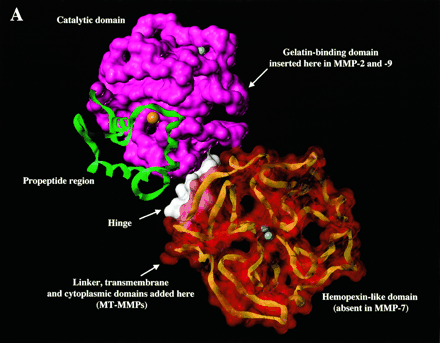
Most members of the MMP family are organized into three basic, distinctive, and well-conserved domains based on structural considerations: an amino-terminal propeptide; a catalytic domain; and a hemopexin-like domain at the carboxy-terminal. The propeptide consists of approximately 80–90 amino acids containing a cysteine residue, which interacts with the catalytic zinc atom via its side chain thiol group. A highly conserved sequence (. . .PRCGXPD. . .) is present in the propeptide. Removal of the propeptide by proteolysis results in zymogen activation, as all members of the MMP family are produced in a latent form. The catalytic domain contains two zinc ions and at least one calcium ion coordinated to various residues. One of the two zinc ions is present in the active site and is involved in the catalytic processes of the MMPs. The second zinc ion (also known as structural zinc) and the calcium ion are present in the catalytic domain approximately 12 Å away from the catalytic zinc. The catalytic zinc ion is essential for the proteolytic activity of MMPs; the three histidine residues that coordinate with the catalytic zinc are conserved among all the MMPs. Little is known about the roles of the second zinc ion and the calcium ion within the catalytic domain, but the MMPs are shown to possess high affinities for structural zinc and calcium ions. The hemopexin-like domain of MMPs is highly conserved and shows sequence similarity to the plasma protein, hemopexin. The hemopexin-like domain has been shown to play a functional role in substrate binding and/or in interactions with the tissue inhibitors of metalloproteinases (TIMPs), a family of specific MMP protein inhibitors. In addition to these basic domains, the family of MMPs evolved into different subgroups by incorporating and/or deleting structural and functional domains. For example, MMP-2 and MMP-9, also known as gelatinases, incorporated the three repeats homologous to the type-II module of fibronectin into the catalytic domain that has been shown to be involved in binding to denatured collagen or gelatin. This domain, known as the gelatin binding domain or fibronectin type-II-like domain, is unique to the gelatinases, and so these enzymes are regarded as a separate subgroup among members of the MMP family. Incorporation of a hydrophobic stretch of approximately 25 amino acids, representing a putative transmembrane domain at the carboxy terminus and recognition motif (RXKR) for furin-like convertases at the end of the propeptide domain, is a characteristic of the membrane-type MMPs (MT-MMPs) except MT4-MMP (vide infra). MMP-11 also contains this furin recognition motif and, similar to the MT-MMPs, it is processed into the active form intracellularly. Additional insertion to the three basic MMP domains also includes a proline-rich 54 amino acid insertion in MMP-9 with sequence similarity to the α2 chain of collagen V. Finally, MMP-7 lacks the hemopexin-like domain and represents the smallest member of the MMP family.[13]
References
- ↑ "Entrez Gene: MMP3 matrix metallopeptidase 3 (stromelysin 1, progelatinase)".
- ↑ "Anti-MMP-3 antibody".
- ↑ 3.0 3.1 3.2 Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM (May 1996). "Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression". The Journal of Biological Chemistry 271 (22): 13055–60. doi:10.1074/jbc.271.22.13055. PMID 8662692.
- ↑ 4.0 4.1 Eguchi T, Kubota S, Kawata K, Mukudai Y, Uehara J, Ohgawara T et al. (Apr 2008). "Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene" (PDF). Molecular and Cellular Biology 28 (7): 2391–2413. doi:10.1128/MCB.01288-07. PMID 18172013.
- ↑ Matrisian LM (Apr 1990). "Metalloproteinases and their inhibitors in matrix remodeling". Trends in Genetics 6 (4): 121–5. doi:10.1016/0168-9525(90)90126-Q. PMID 2132731.
- ↑ Ye S, Watts GF, Mandalia S, Humphries SE, Henney AM (Mar 1995). "Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis". British Heart Journal 73 (3): 209–15. doi:10.1136/hrt.73.3.209. PMC 483800. PMID 7727178.
- ↑ Terashima M, Akita H, Kanazawa K, Inoue N, Yamada S, Ito K et al. (Jun 1999). "Stromelysin promoter 5A/6A polymorphism is associated with acute myocardial infarction". Circulation 99 (21): 2717–9. doi:10.1161/01.cir.99.21.2717. PMID 10351963.
- ↑ Yoon S, Tromp G, Vongpunsawad S, Ronkainen A, Juvonen T, Kuivaniemi H (Nov 1999). "Genetic analysis of MMP3, MMP9, and PAI-1 in Finnish patients with abdominal aortic or intracranial aneurysms". Biochemical and Biophysical Research Communications 265 (2): 563–8. doi:10.1006/bbrc.1999.1721. PMID 10558909.
- ↑ Humphries SE, Luong LA, Talmud PJ, Frick MH, Kesäniemi YA, Pasternack A et al. (Jul 1998). "The 5A/6A polymorphism in the promoter of the stromelysin-1 (MMP-3) gene predicts progression of angiographically determined coronary artery disease in men in the LOCAT gemfibrozil study. Lopid Coronary Angiography Trial". Atherosclerosis 139 (1): 49–56. doi:10.1016/S0021-9150(98)00053-7. PMID 9699891.
- ↑ de Maat MP, Jukema JW, Ye S, Zwinderman AH, Moghaddam PH, Beekman M et al. (Mar 1999). "Effect of the stromelysin-1 promoter on efficacy of pravastatin in coronary atherosclerosis and restenosis". The American Journal of Cardiology 83 (6): 852–6. doi:10.1016/S0002-9149(98)01073-X. PMID 10190398.
- ↑ Letra A, Silva RA, Menezes R, Astolfi CM, Shinohara A, de Souza AP et al. (Oct 2007). "MMP gene polymorphisms as contributors for cleft lip/palate: association with MMP3 but not MMP1". Archives of Oral Biology 52 (10): 954–60. doi:10.1016/j.archoralbio.2007.04.005. PMID 17537400.
- ↑ Bueno DF, Sunaga DY, Kobayashi GS, Aguena M, Raposo-Amaral CE, Masotti C et al. (Jun 2011). "Human stem cell cultures from cleft lip/palate patients show enrichment of transcripts involved in extracellular matrix modeling by comparison to controls". Stem Cell Reviews 7 (2): 446–457. doi:10.1007/s12015-010-9197-3. PMC 3073041. PMID 21052871.
- ↑ Massova I, Kotra LP, Fridman R, Mobashery S (Sep 1998). "Matrix metalloproteinases: structures, evolution, and diversification". FASEB Journal 12 (12): 1075–95. doi:10.1142/S0217984998001256. PMID 9737711.
Further reading
- Matrisian LM (Apr 1990). "Metalloproteinases and their inhibitors in matrix remodeling". Trends in Genetics 6 (4): 121–5. doi:10.1016/0168-9525(90)90126-Q. PMID 2132731.
- Massova I, Kotra LP, Fridman R, Mobashery S (Sep 1998). "Matrix metalloproteinases: structures, evolution, and diversification". FASEB Journal 12 (12): 1075–95. doi:10.1142/S0217984998001256. PMID 9737711.
- Nagase H, Woessner JF (Jul 1999). "Matrix metalloproteinases". The Journal of Biological Chemistry 274 (31): 21491–4. doi:10.1074/jbc.274.31.21491. PMID 10419448.
- Lijnen HR (Jan 2002). "Matrix metalloproteinases and cellular fibrinolytic activity". Biochemistry. Biokhimii͡A 67 (1): 92–8. doi:10.1023/A:1013908332232. PMID 11841344.
External links
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||