MK-2048
 | |
| Systematic (IUPAC) name | |
|---|---|
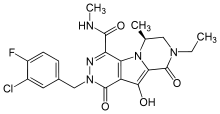
| (6S)-2-(3-chloro-4-fluorobenzyl)-8-ethyl-10-hydroxy-N,6-dimethyl-1,9-dioxo-1,2,6,7,8,9-hexahydropyrazino[1′,2′:1,5]pyrrolo[2,3-d]pyridazine-4-carboxamide | |
| Clinical data | |
| |
| Identifiers | |
| None | |
| PubChem | CID 11554427 |
| ChemSpider | 25058456 |
| ChEMBL | CHEMBL1237018 |
| Chemical data | |
| Formula | C21H21ClFN5O4 |
| 461.87 g/mol | |
|
SMILES
| |
| |
MK-2048 is a second generation integrase inhibitor, intended to be used against HIV infection. It is superior to the first available integrase inhibitor, raltegravir, in that it inhibits the HIV enzyme integrase 4 times longer. It is being investigated for use as part of pre-exposure prophylaxis (PrEP). [1]
It is being developed by Merck & Co.[2]
References
- ↑ Keith Alcorn. Ralvetgravir shows potential for use as PrEP drug AIDSmap.com. 28 April 2009. Accessed 8 Nov 2009.
- ↑ Mark Mascolini. Merck Offers Unique Perspective on Second-Generation Integrase Inhibitor. 10th International Workshop on Clinical Pharmacology of HIV Therapy, April 15–17, 2009, Amsterdam. Accessed 8 Nov 2009.