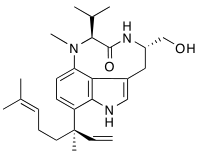
Lyngbyatoxin-a
 | |
| Names | |
|---|---|
| IUPAC name
(4S,7S)-11-((R)-3,7-dimethylocta-1,6-dien-3-yl)-4-(hydroxymethyl)-7-isopropyl-8-methyl-4,5,7,8-tetrahydro-1H-[1,4]diazonino[7,6,5-cd]indol-6(3H)-one | |
| Other names
Lyngbyatoxin-a | |
| Identifiers | |
| 70497-14-2 | |
| Jmol-3D images | Image |
| PubChem | 91706 |
| |
| Properties | |
| Molecular formula |
C27H39N3O2 |
| Molar mass | 437.62 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Lyngbyatoxin-a is a cyanotoxin produced by certain cyanobacteria species, notably Moorea producens (formerly classified as Lyngbya majuscula). It is used as a defensive secretion to protect this cyanobacteria from predation by fish, being a potent irritant and vesicant, as well as a carcinogen. Low concentrations more commonly encountered cause milder symptoms, known as seaweed dermatitis.[1][2][3][4][5][6]
References
- ↑ Cardellina JH 2nd, Marner FJ, Moore RE. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979 Apr 13;204(4389):193-5. PMID 107586
- ↑ Fujiki H, Mori M, Nakayasu M, Terada M, Sugimura T, Moore RE. Indole alkaloids: dihydroteleocidin B, teleocidin, and lyngbyatoxin A as members of a new class of tumor promoters. Proceedings of the National Academy of Sciences USA. 1981 Jun;78(6):3872-6. PMID 6791164
- ↑ Kozikowski AP, Shum PW, Basu A, Lazo JS. Synthesis of structural analogues of lyngbyatoxin A and their evaluation as activators of protein kinase C. Journal of Medicinal Chemistry. 1991 Aug;34(8):2420-30. PMID 1875340
- ↑ Osborne NJ, Webb PM, Shaw GR. The toxins of Lyngbya majuscula and their human and ecological health effects. Environment International. 2001 Nov;27(5):381-92. PMID 11757852
- ↑ Ito E, Satake M, Yasumoto T. Pathological effects of lyngbyatoxin A upon mice. Toxicon. 2002 May;40(5):551-6. PMID 11821127
- ↑ Edwards DJ, Gerwick WH. Lyngbyatoxin biosynthesis: sequence of biosynthetic gene cluster and identification of a novel aromatic prenyltransferase. Journal of the American Chemical Society. 2004 Sep 22;126(37):11432-3. PMID 15366877