Lower flammable limit
Lower flammability limit (LFL),[1] usually expressed in volume per cent, is the lower end of the concentration range over which a flammable mixture of gas or vapour in air can ignite at a given temperature and pressure. The flammability range is delineated by the upper and lower flammability limits. Outside this range of air/vapor mixtures, the mixture will not ignite (unless the temperature and pressure are increased). The LFL decreases with increasing temperature; thus, a mixture that is below its LFL at a given temperature may ignite if heated sufficiently. For liquids, the LFL is typically close to the saturated vapor concentration at the flash point, however, due to differences in the liquid properties, the relationship of LFL to flash point (which is also dependent on the test apparatus) is not fixed and some spread in the data usually exists.
The 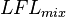 of a mixture can be evaluated using the Le Chatelier mixing rule, if the
of a mixture can be evaluated using the Le Chatelier mixing rule, if the 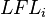 of the components
of the components 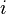 are known:[2]
are known:[2]
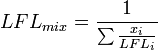
Where 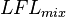 is the lower flammability of the mixture,
is the lower flammability of the mixture, 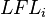 is the lower flammability of the
is the lower flammability of the 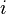 -th component of the mixture, and
-th component of the mixture, and 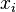 is the molar fraction of the
is the molar fraction of the 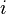 -th component of the mixture.
-th component of the mixture.
See also
- Flammability limit
- Flammability
- Flash point
- Minimum Ignition Energy
- Petroleum
- Stoichiometry
- Upper flammable limit
References
- ↑ 1. ASTM E681-04 http://www.astm.org/Standards/E681.htm
- ↑ Le Chatelier, H. Notes sur le dosage du grisou par les limites d’inflammabilité. Ann. Mines 388–395 (1891).