List of thermal conductivities
In heat transfer, the thermal conductivity of a substance, k, is an intensive property that indicates its ability to conduct heat.
Thermal conductivity is often measured with laser flash analysis. Alternative measurements are also established.
Mixtures may have variable thermal conductivities due to composition.
Note that this table shows thermal conductivity in units of watts per meter per Kelvin (W·m−1·K−1). This is the current metric unit of measurement. Prior to this, however, thermal conductivity was measured in terms of BTUs per foot per hour per degree Fahrenheit. A value for the former can be computed from the latter by multiplying by 1.728.[1]
| Material | Thermal conductivity [W·m−1·K−1] | Temperature [K] | Electrical conductivity @ 293 K [Ω−1·m−1] |
Notes |
|---|---|---|---|---|
| Acrylic Glass (Plexiglas V045i) | 0.17[2]-0.19[2]-0.2[3] | 296[2] | 7.1430001120969E-15[2] - 4.9999999811584E-14[2] | |
| Air | 0.024[4][5][6]-0.025[7] 0.0262 (1 bar)[8] 0.0457 (1 bar)[8] |
273[4][5]-293[7]-298[6] 300[8] 600[8] |
hiAerosols2.95[9]-loAerosols7.83[9]×10−15 | (78.03%N2,21%O2,+0.93%Ar,+0.04%CO2) (1 atm) |
| Alcohols OR Oils | 0.1[6][7]-0.110[10]-0.21[6][7]-0.212[10] | 293[7]-298[6]-300[10] | ||
| Aluminium, pure | 204.3[11]-205[4]-220[12]-237[7][13][14][15]-250[6] 214.6[11] 249.3[11] |
293[7][11]-298[6][14][15] 366[11] 478[11] |
37,450,000[14] - 37,740,000[16] | |
| Aluminium nitride | 170[13]-175[17]-190[17] | 293[17] | 1×10−11[17] | |
| Aluminium oxide, pure | 26[18]-30[7]-35[18]-39[13]-40[19] | 293[7][18][19] | 1×10−12-[18][19] | |
| Ammonia, saturated | 0.507[10] | 300[10] | ||
| Argon | 0.016[6]-0.01772[15]-0.0179[15][20] | 298[6][15]-300[15][20] | ||
| Beryllium oxide | 218[13]-260[21]-300[21] | 293[21] | 1×10−12[21] | |
| Bismuth | 7.97[15] | 300[15] | ||
| Brass Cu63% | 125[22] | 296[22] | 15,150,000[22] - 16,130,000[22] | (Cu63%, Zn37%) |
| Brass Cu70% | 109[4][23] - 121[23] | 293[4]-296[23] | 12,820,000[23] - 16,130,000[23] | (Cu70%, Zn30%) |
| Brick | 0.15[4]-0.6[4]-0.69[6]-1.31[6] | 293[4]-298[6] | ||
| Bronze | 26[12] 42[24]-50[11][24] |
293[11]-296[24] | 5,882,000[24] - 7,143,000[24] |
Sn25%[12] (Cu89%, Sn11%)[24] |
| Calcium silicate | 0.063[25] | 373[25] | ||
| Carbon dioxide | 0.0146[6]-0.01465[26]-0.0168[20](sat. liquid 0.087[27]) | 298[6]-273[26]-300[20](293[27]) | ||
| Carbon nanotubes, bulk | 2.5 (multiwall)[28] - 35 (single wall, disordered mats)[29] - 200(single wall, aligned mats)[30] | 300[31] | "bulk" refers to a group of nanotubes either arranged or disordered, for a single nanotube, see "carbon nanotube, single" .[32] | |
| Carbon nanotube, single | 3180 (multiwall)[33][34]-3500 (single wall)[35] (SWcalc.6,600[33][36]-37,000[33][36]) |
320[33][34]-300[35] (300[33][36]-100[33][36]) |
(Lateral)10−16[37] - (Ballistic)108[37]) | values only for one single SWNT(length:2.6 μm, diameter:1.7 nm) and CNT. "Single", as opposed to "bulk" quantity (see "carbon nanotubes, bulk" ) of many nanotubes, which should not be confused with the denomination of nanotubes themselves which can be singlewall(SWNT) or multiwall(CNT)[38] |
| Concrete | 0.8[4] - 1.28[7] - 1.65 [39] - 2.5 [39] | 293[7] | ~61-67%CaO | |
| Copper, pure | 385[4]-386[11][12]-390[7]-401[6][15][40] 368.7[11] 353.1[11] |
293[4][6][7][11][15][40] 573[11] 873[11] |
59,170,000[40] - 59,590,000[16] | International Annealed Copper Standard (IACS) pure =1.7×10−8Ω•m =58.82×106Ω−1•m−1 For main article, see: Copper in heat exchangers. |
| Cork | 0.04[4] - 0.07[7] | 293[7] | ||
| Cotton or Plastic Insulation-foamed | 0.03[6][7] | 293[7] | ||
| Diamond, impure | 1,000[4][41] | 273[41] - 293[4] | 1×10−16~[42] | Type I (98.1% of Gem Diamonds) (C+0.1%N) |
| Diamond, natural | 2,200[43] | 293[43] | 1×10−16~[42] | Type IIa (99%12C and 1%13C) |
| Diamond, isotopically enriched | 3,320[43]-41,000[33][44](99.999% 12C calc.200,000[44]) | 293[43]-104[33][44](~80[44]) | (Lateral)10−16[42] - (Ballistic)108[42] | Type IIa isotopically enriched (>99.9%12C) |
| Epoxy, thermally conductive | 0.682[45] - 1.038 - 1.384[46] | |||
| Expanded polystyrene - EPS | 0.03[6]-0.033[4][6][41]((PS Only)0.1[47]-0.13[47]) | 98[41]-298[6][41](296[47]) | 1×10−14[47] | (PS+Air+CO2+CnH2n+x) |
| Extruded polystyrene - XPS | 0.029 - 0.39 | 98-298 | ||
| Fiberglass or Foam-glass | 0.045[7] | 293[7] | ||
| Gallium arsenide | 56[41] | 300[41] | ||
| Glass | 0.8[4]-0.93[7](SiO2pure1[13]-SiO296%1.2[48]-1.4[48]) | 293[4][7][48] | 10−14[49][50]-10−12[48]-10−10[49][50] | <1% Iron oxides |
| Glycerol | 0.285[10]-0.29[7] | 300[10]-293[7] | ||
| Gold, pure | 314[4]-315[11]-318[12][15][51] | 293[11]-298[15][51] | 45,170,000[16] - 45,450,000[51] | |
| Granite | 1.73[52] - 3.98[52] | (72%SiO2+14%Al2O3+4%K2O etc.) | ||
| Graphene | (4840±440)[53] - (5300±480)[53] | 293[53] | 100,000,000[54] | |
| Graphite, natural | 25-470[55] | 293[55] | 5000000-30000000[55] | |
| Helium II | >100000[56] | 2.2 | liquid Helium in its superfluid state below 2.2 K | |
| Hydrogen | 0.1819[57] | 290 | Hydrogen gas at room temperature. | |
| Ice | 1.6[4]-2.1[7]-2.2[41]-2.22[58] | 293[4][7] - 273[41][58] | ||
| Indium phosphide | 80[41] | 300[41] | ||
| Iron, pure | 71.8[12]-72.7[11]-79.5[4]-80[6]-80.2[41]-80.4[15][59] 55.4[11] 34.6[11] |
293[4][11]-298[6]-300[15][41][59] 573[11] 1273[11] |
9,901,000[59] - 10,410,000[16] | |
| Iron, cast | 55[6][12] | 298[6] | (Fe+(2-4)%C+(1-3)%Si) | |
| Lead, pure | 34.7[4][11]-35.0[6][12]-35.3[15][60] 29.8[11] |
293[4][11]-298[6]-300[15][60] 573[11] |
4,808,000[16] - 4,854,000[60] | |
| Limestone | 1.26[52] - 1.33[52] | Mostly CaCO3 | ||
| Marble | 2.07[52]-2.08[6]-2.94[6][52] | 298[6] | Mostly CaCO3 | |
| Methane | 0.030[6]-0.03281[61] | 298[6]-273[61] | ||
| Mineral Insulation or Wool(Felt/Glass/Rock) | 0.04[4][6][7] | 293[7]-298[6] | ||
| Nickel | 90.9[15]-91[6] | 298[6][15] | ||
| Nitrogen, pure | 0.0234[4]-0.024[6]-0.02583[15]-0.026[20][41] | 293[4]-298[6]-300[15][20][41] | (N2) (1 atm) | |
| Oxygen, pure (gas) | 0.0238[4]-0.024[6]-0.0263[20]-0.02658[15] | 293[4]-298[6]-300[15][20] | (O2) (1 atm) | |
| Paper | 0.05[6] | 298[6] | ||
| Perlite, (1 atm) | 0.031[6] | 298[6] | ||
| Perlite in partial vacuum | 0.00137[6] | 298[6] | ||
| Plastic, fiber-reinforced | 0.23[62] - 0.7[62] - 1.06[7] | 293[7] - 296[62] | 10−15[62] - 100[62] | 10-40%GF or CF |
| Polyethylene High Density | 0.42[6] - 0.51[6] | 298[6] | ||
| Polymer, High-Density | 0.33[62] - 0.52[62] | 296[62] | 10−16[62] - 102[62] | |
| Polymer, Low-density | 0.04[62] - 0.16[7] - 0.25[7] - 0.33[62] | 293[7] - 296[62] | 10−17[62] - 100[62] | |
| Polyurethane foam | 0.02[6] - 0.021[6] | 298[6] | ||
| Quartz (single crystal) | 12[41] 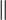 to c axis, 6.8[41] to c axis, 6.8[41] 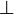 to c axis to c axis |
300[41] | ||
| Quartz-Fused or Vitreous Silica or Fused Silica | 1.46[63]-3[7] 1.4[41] |
293[7][63] 323[41] |
1.3329999732855E-18[49] - 10−16[63] | |
| Rice hulls (ash) | 0.062[64] | |||
| Rice hulls (whole) | 0.0359[64] | |||
| Rubber (92%) | 0.16[41] | 303[41] | 1×10−13~[49] | |
| Sandstone | 1.83[52] - 2.90[52] 2.1[65] - 3.9[65] |
~95-71%SiO2 ~98-48%SiO2, ~16-30% Porosity | ||
| Silica Aerogel | 0.003[41](carbon black9%~0.0042[66])-0.008[66]-0.017[66]-0.02[6]-0.03[41] | 98[41] - 298[6][41] | Foamed Glass | |
| Silver, pure | 406[4]-407[11]-418[12] 427[13]-429[6][15][41][67]-430[15] |
293[4][11] 298[6][15][67]-300[15][41] |
61,350,000[67] - 63,010,000[16] | Highest electrical conductivity of any metal |
| Silver, sterling | 361[68] | |||
| Snow, dry | 0.05[6]-0.11[4]-0.25[6] | 273[6] | ||
| Sodium chloride | 35.1 - 6.5 - 4.85[69] | 80 - 289 - 400[69] | ||
| Soil, dry w/ organic matter | 0.15[7][70]-1.15[70]-2[7] | 293[7] | composition may vary | |
| Soil, saturated | 0.6[7]-4[7] | 293[7] | composition may vary | |
| Solder, Sn/63% Pb/37% | 50[71] | |||
| Lead free solder, Sn/95.6% Ag/3.5% Cu/0.9%, Sn/95.5% Ag/3.8% Cu/0.7% (SAC) | ~60[71] | |||
| Steel, carbon | 36[11][12]-43[6]50.2[4]-54[6][11][12] | 293[4][11]-298[6] | (Fe+(1.5-0.5)%C) | |
| Steel, stainless | 16.3[12][72]-16.7[73]-18[74]-24[74] | 296[72][73][74] | 1,176,000[73] - 1,786,000[74] | (Fe, Cr12.5-25%, Ni0-20%, Mo0-3%, Ti0-trace) |
| Thermal grease, silver-based | 0.94+[75] | |||
| Thermal tape | 0.60[76] | |||
| Titanium, pure | 15.6[12]-19.0[11]-21.9[15][77]-22.5[11] | 293[11]-300[15][77] | 1,852,000[77] - 2,381,000[16] | |
| Titanium Alloy | 5.8[78] | 296[78] | 595,200[78] | (Ti+6%Al+4%V) |
| Tungsten, Pure | 173[42] | 293[42] | 18,940,000[42] | |
| Water | 0.563[79]-0.596[79]-0.6[4][7]-0.609[10] | 273[79]-293[4][7][79]-300[10] | 5×Pure10−6[42]-Sweet10−3±1[42]-Sea1[79] | <4[79]%(NaCl+MgCl2+CaCl2) |
| Water vapor | 0.016[6]-0.02479 (101.3 kPa)[80] 0.0471 (1 bar)[8] |
293[80]-398[6] 600[8] |
||
| Wood, +>=12% water | 0.09091[81]-0.16[41]-0.21[81]-0.4[7] | 298[41]-293[7] | Species-Variable[81] | |
| Wood, oven-dry | 0.04[4]-0.055[6]-0.07692[81]-0.12[4]-0.17[6][81] | 293[4]-298[6] | Balsa[6]-Cedar[81]-Hickory[81]/Oak[6] | |
| Zinc, Pure | 116[42] | 293[42] | 16,950,000[42] | |
| Zinc oxide | 21[13] | |||
| Material | Thermal conductivity [W·m−1·K−1] | Temperature [K] | Electrical conductivity @ 293 K [Ω−1·m−1] | Notes |
See also
- Laser flash analysis
- List of insulation material
- R-value
- Specific heat capacity
- Thermal conductivity
- Thermal conductivities of the elements (data page)
- Thermal diffusivity
References
- ↑ Roger N. Wright (3 December 2010). "Wire Technology: Process Engineering and Metallurgy". Elsevier. p. 281. ISBN 978-0-12-382093-8{{inconsistent citations}}
- ↑ 2.0 2.1 2.2 2.3 2.4 http://www.goodfellow.com/E/Polymethylmethacrylate.html
- ↑ http://www.plexiglas.com/tds/4b.pdf
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 4.20 4.21 4.22 4.23 4.24 4.25 4.26 4.27 4.28 4.29 4.30 4.31 4.32 4.33 4.34 4.35 4.36 4.37 4.38 HyperPhysics, most from Young, Hugh D., University Physics, 7th Ed., Addison Wesley, 1992. Table 15-5. (most data should be at 293 K (20 °C; 68 °F))
- ↑ 5.0 5.1 http://www.engineeringtoolbox.com/air-properties-d_156.html
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 6.9 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 6.18 6.19 6.20 6.21 6.22 6.23 6.24 6.25 6.26 6.27 6.28 6.29 6.30 6.31 6.32 6.33 6.34 6.35 6.36 6.37 6.38 6.39 6.40 6.41 6.42 6.43 6.44 6.45 6.46 6.47 6.48 6.49 6.50 6.51 6.52 6.53 6.54 6.55 6.56 6.57 6.58 6.59 6.60 6.61 6.62 6.63 6.64 6.65 6.66 6.67 http://www.engineeringtoolbox.com/thermal-conductivity-d_429.html
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 7.9 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 7.20 7.21 7.22 7.23 7.24 7.25 7.26 7.27 7.28 7.29 7.30 7.31 7.32 7.33 7.34 7.35 7.36 7.37 7.38 7.39 7.40 7.41 7.42 7.43 Hukseflux Thermal Sensors
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 "Thermal conductivity of gases", CRC Handbook, p. 6–195.
- ↑ 9.0 9.1 Pawar, S. D.; Murugavel, P.; Lal, D. M. (2009). "Effect of relative humidity and sea level pressure on electrical conductivity of air over Indian Ocean". Journal of Geophysical Research 114: D02205. Bibcode:2009JGRD..11402205P. doi:10.1029/2007JD009716.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 http://www.engineeringtoolbox.com/thermal-conductivity-liquids-d_1260.html
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 11.9 11.10 11.11 11.12 11.13 11.14 11.15 11.16 11.17 11.18 11.19 11.20 11.21 11.22 11.23 11.24 11.25 11.26 11.27 11.28 11.29 11.30 11.31 11.32 11.33 http://www.engineeringtoolbox.com/thermal-conductivity-metals-d_858.html
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 12.9 12.10 12.11 12.12 http://www.engineersedge.com/properties_of_metals.htm
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 Greg Becker, Chris Lee, and Zuchen Lin (July 2005). "Thermal conductivity in advanced chips — Emerging generation of thermal greases offers advantages". Advanced Packaging: pp.2–4. Retrieved 2008-03-04.
- ↑ 14.0 14.1 14.2 http://www.goodfellow.com/E/Aluminium.html
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 15.8 15.9 15.10 15.11 15.12 15.13 15.14 15.15 15.16 15.17 15.18 15.19 15.20 15.21 15.22 15.23 15.24 15.25 15.26 15.27 Thermal conductivities of the elements (data page)
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 Electrical resistivities of the elements (data page)
- ↑ 17.0 17.1 17.2 17.3 http://www.goodfellow.com/E/AluminiumNitride'.html
- ↑ 18.0 18.1 18.2 18.3 http://www.goodfellow.com/E/Alumina.html
- ↑ 19.0 19.1 19.2 Alumina (Al2O3) - Physical, Mechanical, Thermal, Electrical and Chemical Properties - Supplier Data by Ceramaret
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 http://www.engineersedge.com/heat_transfer/thermal-conductivity-gases.htm
- ↑ 21.0 21.1 21.2 21.3 http://www.goodfellow.com/E/Beryllia.html
- ↑ 22.0 22.1 22.2 22.3 http://www.goodfellow.com/E/Brass.html
- ↑ 23.0 23.1 23.2 23.3 23.4 http://www.goodfellow.com/E/Brass'.html
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 http://www.goodfellow.com/E/Bronze.html
- ↑ 25.0 25.1 http://www.engineeringtoolbox.com/calcium-silicate-insulation-k-values-d_1171.html
- ↑ 26.0 26.1 http://encyclopedia.airliquide.com/encyclopedia.asp?GasID=26
- ↑ 27.0 27.1 http://www.engineeringtoolbox.com/carbon-dioxide-d_1000.html
- ↑ "Carbon nanotubes : Reinforced metal matrix composites" by A.Agarwal, S.R.Bakshi and D.Lahiri, CRC Press, 2011 (ch.1, p.8, chart 1.1 : physical properties of carbon materials )
- ↑ "Carbon nanotubes : Reinforced metal matrix composites" by A.Agarwal, S.R.Bakshi and D.Lahiri, CRC Press, 2011 (ch.1, p.8, chart 1.1 : physical properties of carbon materials )
- ↑ "Carbon nanotubes : Reinforced metal matrix composites" by A.Agarwal, S.R.Bakshi and D.Lahiri, CRC Press, 2011 (ch.1, p.8, chart 1.1 : physical properties of carbon materials )
- ↑ "Carbon nanotubes : Reinforced metal matrix composites" by A.Agarwal, S.R.Bakshi and D.Lahiri, CRC Press, 2011 (ch.1, p.8, chart 1.1 : physical properties of carbon materials )
- ↑ "Carbon nanotubes : Reinforced metal matrix composites" by A.Agarwal, S.R.Bakshi and D.Lahiri, CRC Press, 2011 (ch.1, p.8, chart 1.1 : physical properties of carbon materials )
- ↑ 33.0 33.1 33.2 33.3 33.4 33.5 33.6 33.7 "Carbon Nanotubes: Thermal Properties" (PDF). Retrieved 2009-06-06.
- ↑ 34.0 34.1 Kim, P.; Shi, L.; Majumdar, A.; McEuen, P. L. et al. (2001-06-01). "Thermal transport measurements of individual multiwalled nanotubes". Physical Review Letters 87 (21): 215502–215506. arXiv:cond-mat/0106578. Bibcode:2001PhRvL..87u5502K. doi:10.1103/PhysRevLett.87.215502. PMID 11736348.
- ↑ 35.0 35.1 Pop, Eric; Mann, David; Wang, Qian; Goodson, Kenneth; Dai, Hongjie et al. (2005-12-22). "Thermal conductance of an individual single-wall carbon nanotube above room temperature". Nano Letters 6 (1): 96–100. arXiv:cond-mat/0512624. Bibcode:2006NanoL...6...96P. doi:10.1021/nl052145f. PMID 16402794.
- ↑ 36.0 36.1 36.2 36.3 Berber, Savas; Kwon, Young-Kyun; Tománek, David (2000-02-23). "Unusually high thermal conductivity of carbon nanotubes". Physical Review Letters 84 (20): 4613–4616. arXiv:cond-mat/0002414. Bibcode:2000PhRvL..84.4613B. doi:10.1103/PhysRevLett.84.4613. PMID 10990753.
- ↑ 37.0 37.1 Li, Qingwen; Li, Yuan; Chikkannanavar, S. B.; Zhao, Y. H.; Dangelewicz, A. M.; Zheng, L. X.; Doorn, S. K. et al. (2007). "Structure-Dependent Electrical Properties of Carbon Nanotube Fibers". Advanced Materials 19 (20): 3358–3363. doi:10.1002/adma.200602966.
- ↑ "Carbon nanotubes : Reinforced metal matrix composites" by A.Agarwal, S.R.Bakshi and D.Lahiri, CRC Press, 2011 (ch.1, p.8, chart 1.1 : physical properties of carbon materials )
- ↑ 39.0 39.1 International Standard EN-ISO 10456:2007 'Building materials and products - Hygrothermal properties - Tabulated design values and procedures for determining declared and design thermal values'
- ↑ 40.0 40.1 40.2 http://www.goodfellow.com/E/Copper.html
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 41.6 41.7 41.8 41.9 41.10 41.11 41.12 41.13 41.14 41.15 41.16 41.17 41.18 41.19 41.20 41.21 41.22 41.23 41.24 41.25 41.26 41.27 41.28 41.29 CRC handbook of chemistry and physics(subscription required)(HTTP cookies required)
- ↑ 42.0 42.1 42.2 42.3 42.4 42.5 42.6 42.7 42.8 42.9 42.10 42.11 Other references listed within Wikipedia (this table may not be cited, pure elements are sourced from Chemical elements data references, otherwise an in-table linked-page must list the relevant references)
- ↑ 43.0 43.1 43.2 43.3 Anthony, T. R.; Banholzer, W. F.; Fleischer, J. F.; Wei, Lanhua; Kuo, P. K.; Thomas, R. L.; Pryor, R. W. (1989-12-27). "Thermal conductivity of isotopically enriched 12C diamond". Physical Review B 42 (2): 1104–1111. Bibcode:1990PhRvB..42.1104A. doi:10.1103/PhysRevB.42.1104.
- ↑ 44.0 44.1 44.2 44.3 Wei, Lanhua; Kuo, P. K.; Thomas, R. L.; Anthony, T. R.; Banholzer, W. F. (1993-02-16). "Thermal conductivity of isotopically modified single crystal diamond". Physical Review Letters 70 (24): 3764–3767. Bibcode:1993PhRvL..70.3764W. doi:10.1103/PhysRevLett.70.3764. PMID 10053956.
- ↑ "MG 832TC Thermally Conductive Epoxy".
- ↑ "OMEGABOND OB-100/101/200 Thermally Conductive Epoxies" (PDF).
- ↑ 47.0 47.1 47.2 47.3 http://www.goodfellow.com/E/Polystyrene.html
- ↑ 48.0 48.1 48.2 48.3 http://www.goodfellow.com/E/Silica.html
- ↑ 49.0 49.1 49.2 49.3 Serway, Raymond A. (1998). Principles of Physics (2nd ed.). Fort Worth, Texas; London: Saunders College Pub. p. 602. ISBN 0-03-020457-7.
- ↑ 50.0 50.1 Griffiths, David (1999) [1981]. "7. Electrodynamics". In Alison Reeves (ed.). Introduction to Electrodynamics (3rd ed.). Upper Saddle River, New Jersey: Prentice Hall. p. 286. ISBN 0-13-805326-X. OCLC 40251748.
- ↑ 51.0 51.1 51.2 http://www.goodfellow.com/E/Gold.html
- ↑ 52.0 52.1 52.2 52.3 52.4 52.5 52.6 52.7 Marble Institute of America (2 values are usually given: the highest and lowest test scores)
- ↑ 53.0 53.1 53.2 Balandin, Alexander A.; Ghosh, Suchismita; Bao, Wenzhong; Calizo, Irene; Teweldebrhan, Desalegne; Miao, Feng; Lau, Chun Ning et al. (2008-02-20). "Superior Thermal Conductivity of Single-Layer Graphene". Nano Letters ASAP 8 (3): 902–907. Bibcode:2008NanoL...8..902B. doi:10.1021/nl0731872. PMID 18284217.
- ↑ Physicists Show Electrons Can Travel More Than 100 Times Faster in Graphene
- ↑ 55.0 55.1 55.2 Properties of Graphite
- ↑ Clifford A. Hampel (1968). The Encyclopedia of the Chemical Elements. New York: Van Nostrand Reinhold. pp. 256–268. ISBN 0-442-15598-0.
- ↑ M. J. Assael, S. Mixafendi, W. A. Wakeham (1 July 1986). "The Viscosity and Thermal Conductivity of Normal Hydrogen in the Limit of Zero Density" (PDF). NIST. Retrieved 2 April 2015.
- ↑ 58.0 58.1 http://www.engineeringtoolbox.com/ice-thermal-properties-d_576.html
- ↑ 59.0 59.1 59.2 http://www.goodfellow.com/E/Iron.html
- ↑ 60.0 60.1 60.2 http://www.goodfellow.com/E/Lead.html
- ↑ 61.0 61.1 http://encyclopedia.airliquide.com/Encyclopedia.asp?GasID=41
- ↑ 62.0 62.1 62.2 62.3 62.4 62.5 62.6 62.7 62.8 62.9 62.10 62.11 62.12 62.13 62.14 http://www.goodfellow.com/Home.aspx?LangType=2057
- ↑ 63.0 63.1 63.2 http://www.goodfellow.com/E/Quartz-Fused.html
- ↑ 64.0 64.1 http://esrla.com/pdf/ricehullhouse.pdf
- ↑ 65.0 65.1 http://edoc.gfz-potsdam.de/gfz/get/15306/0/69070f5918278d63d23cfce5cbad024a/15306.pdf
- ↑ 66.0 66.1 66.2 http://energy.lbl.gov/ECS/aerogels/sa-thermal.html Thermal Properties - Silica Aerogels
- ↑ 67.0 67.1 67.2 http://www.goodfellow.com/E/Silver.html
- ↑ S. Vandana (1 December 2002). "Alternative Energy". APH. p. 45. ISBN 978-81-7648-349-0{{inconsistent citations}}
- ↑ 69.0 69.1 http://www.almazoptics.com/NaCl.htm
- ↑ 70.0 70.1 Soil Sci Journals
- ↑ 71.0 71.1 "Thermal Conductivity-of Solders".
- ↑ 72.0 72.1 http://www.goodfellow.com/E/Stainless-Steel-AISI-302.html
http://www.goodfellow.com/E/Stainless-Steel-AISI-304.html
http://www.goodfellow.com/E/Stainless-Steel-AISI-310.html
http://www.goodfellow.com/E/Stainless-Steel-AISI-316.html
http://www.goodfellow.com/E/Stainless-Steel-AISI-321.html - ↑ 73.0 73.1 73.2 http://www.goodfellow.com/E/Stainless-Steel-17-7PH.html
- ↑ 74.0 74.1 74.2 74.3 http://www.goodfellow.com/E/Stainless-Steel-AISI-410.html
- ↑ NREL Review of Thermal Greases (Free PDF Form Available Through Search Engines)
- ↑
- ↑ 77.0 77.1 77.2 http://www.goodfellow.com/E/Titanium.html
- ↑ 78.0 78.1 78.2 http://www.goodfellow.com/E/Titanium-Aluminium-Vanadium.html
- ↑ 79.0 79.1 79.2 79.3 79.4 79.5 "2.7.9 Physical properties of sea water". Kaye and Laby - National Physical Laboratory. Retrieved 2010-01-25.
- ↑ 80.0 80.1 "Thermal conductivity of saturated H2O and D2O, CRC Handbook, p. 6–4.
- ↑ 81.0 81.1 81.2 81.3 81.4 81.5 81.6 "Physical Properties and Moisture Relations of Wood" (PDF).
- David R. Lide, ed. (2003). CRC Handbook of Chemistry and Physics (84th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0484-9.
External links
- Heat Conduction Calculator
- Thermal Conductivity Online Converter - An online thermal conductivity calculator
- Thermal Conductivities of Solders
- Thermal conductivity of air as a function of temperature can be found at James Ierardi's Fire Protection Engineering Site