List of phenyltropanes
Phenyltropanes are a family of chemical compounds originally derived from structural modification of cocaine. These compounds present many different avenues of research into therapeutic applications, particularly in addiction treatment. Uses vary depending on their construction and structure-activity relationship ranging from the treating of cocaine dependency to understanding the dopamine reward system in the human brain to treating Alzheimer's & Parkinson's diseases. More recently there have been continual additions to the list and enumerations of the plethora of types of chemicals that fall into the category of this substance profile. Many of the compounds were first elucidated in published material by the Research Triangle Institute (RTI) and are thus so named. Similarly, a number of others are named for Sterling-Winthrop pharmaceuticals (WIN) & Wake Forest University (WF).

2-Carboxymethyl esters
Certain phenyltropanes can be used as a smoking cessation aid. Blank spacings within tables for omitted data use "no data", "?", "-" or "—" interchangeably.
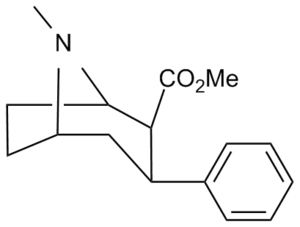
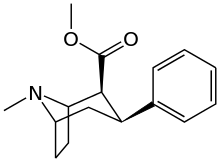
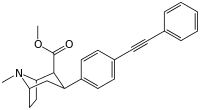
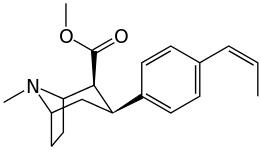
| Short Name | X | DA | 5HT | NE |
|---|---|---|---|---|
| WIN 35,065-2 (CPT) | H | 23 | 1962 | 920 |
| WIN 35,428 (CFT) | F | 14 | 156 | 85 |
| ? | NO2 | 10.1 | ? | ? |
| RTI-29[2] | NH2 | 9.8 | 5110 | 151 |
| RTI-31 | Cl | 1.12 | 44.5 | 37 |
| RTI-32 | Me | 1.71 | 240 | 60 |
| RTI-51 | Br | 1.69 | 10.6 | 37.4 |
| Iometopane (RTI-55) | I | 1.26 | 4.21 | 36 |
| RTI-83 | Et | 55 | 28.4 | 4030 |
| RTI-11w | cis-propenyl | 15 | 7.1 | 28,000 |
| RTI-298[3] | –≡–Ph | 3.7 | 46.8 | 347 |
| RTI-436 | –CH=CHPh | 3.09 | 335 (31) | 1960 (1181) |
| RTI-430 | –C≡C(CH2)2Ph | 6.28 | 2128 (198) | 1470 (886) |
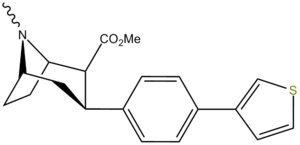
| Tamagnan[1] | p-thiophene | 12 | 0.017 | 189 |
| Compound | Short Name (S. Singh) |
Y | X | DA | 5HT | NE | Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|
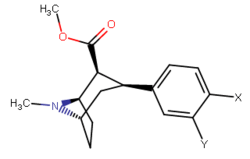 | ||||||||
| 16a | F | H | 23 ± 7.8 | - | - | - | - | |
| 16b | Cl | H | 10.6 ± 1.8 | - | - | - | - | |
| 16c | Br | H | 7.93 ± 0.08 IC50 determined in Cynomolgous monkey caudate-putamen | - | - | - | - | |
| 16d | I | H | 26.1 ± 1.7 | - | - | - | - | |
| 16e | SnBu3 | H | 1100 ± 170 | - | - | - | - | |
| 17a | CH3 | F | 2.95 ± 0.58 | - | - | - | - | |
| 17b | CH3 | Cl | 0.81 ± 0.05 | 10.5 ± 0.05 | 36.2 ± 1.0 | 13.0 | 44.7 | |
| 17c | Cl | Cl | 0.79 ± 0.08 | 3.13 ± 0.36 | 18.0 ± 0.8 | 4.0 | 22.8 | |
| 17d | Br | NH2 | 3.91 ± 0.59 | - | - | - | - | |
| 17e | I | NH2 | 1.35 ± 0.11 | 120 ± 4 | 1329 ± 124 | 88.9 | 984 | |
| 17f | I | N3 | 4.93 ± 0.32 | - | - | - | - |
(3,4-Disubstituted phenyl)-tropanes
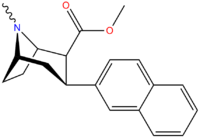
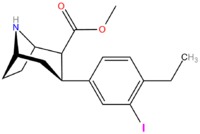
| Compound | X | Y | 2 Position | config | 8 | DA | 5-HT | NE |
| RTI-318 | β-naphthyl | CO2Me | β,β | NMe | 0.5 | 0.81 | 20 | |
| Dichloropane (RTI-111) | Cl | Cl | CO2Me | β,β | NMe | 0.79 | 3.13 | 18.0 |
| RTI-88 [recheck] | NH2 | I | CO2Me | β,β | NMe | 1.35 | 1329 | 320 |
| RTI-97 | NH2 | Br | CO2Me | β,β | NMe | 3.91 | 181 | 282 |
| RTI-112 | Cl | Me | CO2Me | β,β | NMe | 0.82 | 10.5 | 36.2 |
| RTI-96 | F | Me | CO2Me | β,β | NMe | 2.95 | 76 | 520 |
| RTI-295 | Et | I | CO2Me | β,β | NMe | 21.3 | 2.96 | 1349 |
| RTI-353 (EINT) | Et | I | CO2Me | β,β | NH | 331 | 0.69 | 148 |
| RTI-279 | Me | I | CO2Me | β,β | NH | 5.98 | 1.06 | 74.3 |
| RTI-280 | Me | I | CO2Me | β,β | NMe | 3.12 | 6.81 | 484 |
| Meltzer[5] | catechol | CO2Me | β,β | NMe | >100 | ? | ? | |
| Meltzer[5] | OAc | OAc | CO2Me | β,β | NMe | ? | ? | ? |
| Compound | Short Name (S. Singh) |
R | X | IC50 (nM) DAT [3H]WIN 35428 |
IC50 (nM) 5-HTT [3H]paroxetine |
IC50 (nM) NET [3H]nisoxetine |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|
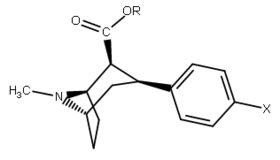 | ||||||||
| 23a | CH(CH3)2 | H | 85.1 ± 2.5 | 23121 ± 3976 | 32047 ± 1491 | 272 | 376 | |
| 23b | C6H5 | H | 76.7 ± 3.6 | 106149 ± 7256 | 19262 ± 593 | 1384 | 251 | |
| 24a | CH(CH3)2 | Cl | 1.4 ± 0.13 6.04 ± 0.31ɑ | 1400 ± 7 128 ± 15b | 778 ± 21 250 ± 0.9c | 1000 21.2d | 556 41.4e | |
| 24b | cyclopropyl | Cl | 0.96 ± 0.10 | 168 ± 1.8 | 235 ± 8.39 | 175 | 245 | |
| 24c | C6H5 | Cl | 1.99 ± 0.05 5.25 ± 0.76ɑ | 2340 ± 27 390 ± 34b | 2960 ± 220 242 ± 30c | 1176 74.3d | 1.3 41.6e | |
| 24d | C6H4-4-I | Cl | 32.6 ± 3.9 | 1227 ± 176 | 967.6 ± 26.3 | 37.6 | 29.7 | |
| 24e | C6H4-3-CH3 | Cl | 9.37 ± 0.52 | 2153 ± 143 | 2744 ± 140 | 230 | 293 | |
| 24f | C6H4-4-CH3 | Cl | 27.4 ± 1.5 | 1203 ± 42 | 1277 ± 118 | 43.9 | 46.6 | |
| 24g | C6H4-2-CH3 | Cl | 3.91 ± 0.23 | 3772 ± 384 | 4783 ± 387 | 965 | 1223 | |
| 24h | C6H4-4-Cl | Cl | 55 ± 2.3 | 16914 ± 1056 | 4883 ± 288 | 307 | 88.8 | |
| 24i | C6H4-4-OCH3 | Cl | 71 ± 5.6 | 19689 ± 1843 | 1522 ± 94 | 277 | 21.4 | |
| 24j | (CH2)2C6H4-4-NO2 | Cl | 2.71 ± 0.13 | - | - | - | - | |
| 24k | (CH)2C6H4-4-NH2 | Cl | 2.16 ± 0.25 | - | - | - | - | |
| 24l | (CH2)2C6H3-3-I-4-NH2 | Cl | 2.51 ± 0.25 | - | - | - | - | |
| 24m | (CH2)2C6H3-3-I-4-N3 | Cl | 14.5 ± 0.94 | - | - | - | - | |
| 24n | (CH2)2C6H4-4-N3 | Cl | 6.17 ± 0.57 | - | - | - | - | |
| 24o | (CH2)2C6H4-4-NCS | Cl | 5.3 ± 0.6 | - | - | - | - | |
| 24p | (CH2)2C6H4-4-NHCOCH2Br | Cl | 1.73 ± 0.06 | - | - | - | - | |
| 25a | CH(CH3)2 | I | 0.43 ± 0.05 2.79 ± 0.13ɑ | 66.8 ± 6.53 12.5 ± 1.0b | 285 ± 7.6 41.2 ± 3.0c | 155 4.5d | 663 14.8e | |
| 25b | cyclopropyl | I | 0.61 ± 0.08 | 15.5 ± 0.72 | 102 ± 11 | 25.4 | 167 | |
| 25c | C6H5 | I | 1.51 ± 0.34 6.85 ± 0.93ɑ | 184 ± 22 51.6 ± 6.2b | 3791 ± 149 32.7 ± 4.4c | 122 7.5d | 2510 4.8e | |
| 26a | CH(CH3)2 | CH3 | 6.45 ± 0.85 15.3 ± 2.08ɑ | 6090 ± 488 917 ± 54b | 1926 ± 38 73.4 ± 11.6c | 944 59.9d | 299 4.8e | |
| 26b | CH(C2H5)2 | CH3 | 19.1 ± 1 | 4499 ± 557 | 3444 ± 44 | 235 | 180 | |
| 26c | cyclopropyl | CH3 | 17.8 ± 0.76 | 485 ± 21 | 2628 ± 252 | 27.2 | 148 | |
| 26d | cyclobutyl | CH3 | 3.74 ± 0.52 | 2019 ± 133 | 4738 ± 322 | 540 | 1267 | |
| 26e | cyclopentyl | CH3 | 1.68 ± 0.14 | 1066 ± 109 | 644 ± 28 | 634 | 383 | |
| 26f | C6H5 | CH3 | 3.27 ± 0.06 9.13 ± 0.79ɑ | 24500 ± 1526 1537 ± 101b | 5830 ± 370 277 ± 23c | 7492 168d | 1783 30.3e | |
| 26g | C6H4-3-CH3 | CH3 | 8.19 ± 0.90 | 5237 ± 453 | 2136 ± 208 | 639 | 261 | |
| 26h | C6H4-4-CH3 | CH3 | 81.2 ± 16 | 15954 ± 614 | 4096 ± 121 | 196 | 50.4 | |
| 26i | C6H4-2-CH3 | CH3 | 23.2 ± 0.97 | 11040 ± 504 | 25695 ± 1394 | 476 | 1107 | |
| 26j | C6H4-4-Cl | CH3 | 117 ± 7.9 | 42761 ± 2399 | 9519 ± 864 | 365 | 81.3 | |
| 26k | C6H4-4-OCH3 | CH3 | 95.6 ± 8.8 | 82316 ± 7852 | 3151 ± 282 | 861 | 33.0 |
ɑKi value for displacement of [3H]DA uptake. bKi value for displacement of [3H]5-HT uptake. cKi value for displacement of [3H]NE uptake. d[3H]5-HT uptake to [3H]DA uptake ratio. e[3H]NE uptake to [3H]DA uptake ratio.
| Compound | Short Name (S. Singh) |
R | X | IC50 (nM) DAT [3H]WIN 35428 |
IC50 (nM) 5-HTT [3H]Paroxetine |
IC50 (nM) NET [3H]Nisoxetine |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|
| 29a | NH2 | CH3 | 41.8 ± 2.45 | 6371 ± 374 | 4398 ± 271 | 152 | 105 | |
| 29b | N(CH2CH3)2 | CH3 | 24.7 ± 1.93 | 33928 ± 2192 | 6222 ± 729 | 1374 | 252 | |
| 29c | N(OCH3)CH3 | CH3 | 2.55 ± 0.43 | 3402 ± 353 | 422 ± 26 | 1334 | 165 | |
| 29d | 4-morpholine | CH3 | 11.7 ± 0.87 | >100000 | 23601 ± 1156 | >8547 | 2017 |
Carboxyaryl
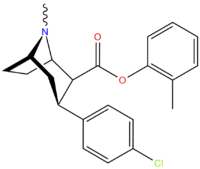
| Compound | X | 2 Position | config | 8 | DA | 5-HT | NE |
| RTI-122 | I | -CO2Ph | β,β | NMe | 1.50 | 184 | 3,791 |
| RTI-113 | Cl | -CO2Ph | β,β | NMe | 1.98 | 2,336 | 2,955 |
| RTI-277 | NO2 | -CO2Ph | β,β | NMe | 5.94 | 2,910 | 5,695 |
| RTI-120 [recheck] | Me | -CO2Ph | β,β | NMe | 3.26 | 24,471 | 5,833 |
| RTI-116 | Cl | -CO2(p-C6H4I) | β,β | NMe | 33 | 1,227 | 968 |
| RTI-203 | Cl | CO2(m-C6H4Me) | β,β | NMe | 9.37 | 2153 | 2744 |
| RTI-204 | Cl | -CO2(o-C6H4Me) | β,β | NMe | 3.91 | 3,772 | 4,783 |
| RTI-205 | Me | -CO2(m-C6H4Me) | β,β | NMe | 8.19 | 5,237 | 2,137 |
| RTI-206 | Cl | -CO2(p-C6H4Me) | β,β | NMe | 27.4 | 1,203 | 1,278 |
Carboxyalkyl
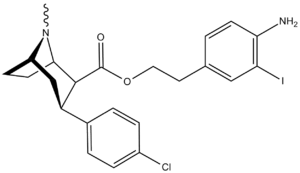
| Code | X | 2 Position | config | 8 | DA | 5-HT | NE |
| RTI-77 | Cl | CH2C2(3-iodo-p-anilino) | β,β | NMe | 2.51 | — | 2247 |
| RTI-121 | I | -CO2Pri | β,β | NMe | 0.43 | 66.8 | 285 |
| RTI-153 | I | -CO2Pri | β,β | NH | 1.06 | 3.59 | 132 |
| RTI-191 | I | -CO2Prcyc | β,β | NMe | 0.61 | 15.5 | 102 |
| RTI-114 | Cl | -CO2Pri | β,β | NMe | 1.40 | 1,404 | 778 |
| RTI-278 | NO2 | -CO2Pri | β,β | NMe | 8.14 | 2,147 | 4,095 |
| RTI-190 | Cl | -CO2Prcyc | β,β | NMe | 0.96 | 168 | 235 |
| RTI-193 | Me | -CO2Prcyc | β,β | NMe | 1.68 | 1,066 | 644 |
| RTI-117 | Me | -CO2Pri | β,β | NMe | 6.45 | 6,090 | 1,926 |
| RTI-150 | Me | -CO2Bucyc | β,β | NMe | 3.74 | 2,020 | 4,738 |
| RTI-127 | Me | -CO2C(H)Et2 | β,β | NMe | 19 | 4500 | 3444 |
| RTI-338 | ethyl | -CO2C2Ph | β,β | NMe | 1104 | 7.41 | 3366 |
Use of a cyclopropyl ester appears to enable better MAT retention than does the choice of isopropyl ester.
Use of a cycBu resulted in greater DAT selectivity than did the cycPr homologue.
Amides
U.S. Patent 5,736,123 RTI-183 and RTI-218 have the same structure??
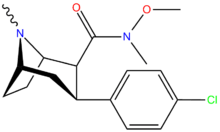
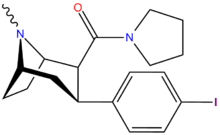
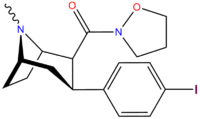
| Code | X | 2 Position | config | 8 | DA | NE | 5-HT |
| RTI-106 | Cl | CON(H)Me | β,β | NMe | 12.4 | 1511 | 1312 |
| RTI-118 | Cl | CONH2 | β,β | NMe | 11.5 | 4267 | 1621 |
| RTI-222 | Me | morpholinyl | β,β | NMe | 11.7 | — | >100K |
| RTI-129 | Cl | CONMe2 | β,β | NMe | 1.38 | 942 | 1079 |
| RTI-146 | Cl | CONHCH2OH | β,β | NMe | 2.05 | 144 | 98 |
| RTI-147 | Cl | CON(CH2)4 | β,β | NMe | 1.38 | 3,949 | 12,394 |
| RTI-156 | Cl | CON(CH2)5 | β,β | NMe | 6.61 | 5832 | 3468 |
| RTI-170 | Cl | CON(H)CH2C≡CH | β,β | NMe | 16.5 | 1839 | 4827 |
| RTI-172 | Cl | CON(H)NH2 | β,β | NMe | 44.1 | 3914 | 3815 |
| RTI-174 | Cl | CONHCOMe | β,β | NMe | 158 | >43K | >125K |
| RTI-182 | Cl | CONHCH2COPh | β,β | NMe | 7.79 | 1722 | 827 |
| RTI-183 | Cl | CON(OMe)Me | β,β | NMe | 0.85 | 549 | 724 |
| RTI-186 | Me | CON(OMe)Me | β,β | NMe | 2.55 | 442 (266) | 3400 (309) |
| RTI-198 | Cl | CON(CH2)3 | β,β | NMe | 6.57 | 990 | 813 |
| RTI-196 | Cl | CONHOMe | β,β | NMe | 10.7 | 9907 | >43K |
| RTI-201 | Cl | CONHNHCOPh | β,β | NMe | 91.8 | >20K | >48K |
| RTI-208 | Cl | CONO(CH2)3 | β,β | NMe | 1.47 | 998 | 2470 |
| RTI-214 | Cl | CON(-CH2CH2-)2O | β,β | NMe | 2.90 | 8545 | >88K |
| RTI-215 | Cl | CONEt2 | β,β | NMe | 5.48 | ? | 9432 |
| RTI-217 | Cl | CONH(m-C6H4OH) | β,β | NMe | 4.78 | >30K | >16K |
| RTI-218 | Cl | CON(Me)OMe | β,β | NMe | 1.19 | 520 | 1911 |
| RTI-226 | Cl | CONMePh | β,β | NMe | 45.0 | ? | 24K |
| RTI-227 | I | CONO(CH2)3 | β,β | NMe | 0.75 | 446 | 230 |
| RTI-229[6] | I | CON(CH2)4 | β,β | NMe | 0.37 | 991 | 1,728 |
Acyl
| # | X | Y | 2 Position | config | 8 | DA | 5-HT | NE |
| WF-23 | β-naphthyl | C(O)Et | β,β | NMe | 0.115 | 0.394 | No data | |
| WF-31 | -Pri | H | C.O.Et | β,β | NMe | 615 | 54.5 | No data |
| WF-11 | Me | H | -C.O.Et | β,β | NMe | 8.2 | 131 | No data |
| WF-25 | H | H | -C.O.Et | β,β | NMe | 48.3 | 1005 | No data |
| WF-33 | 6-MeoBN | C(O)Et | α,β | NMe | 0.13 | 2.24 | No data | |
Ester reduction
Note: p-fluorophenyl is weaker than the others. RTI-145 is not peroxy, it is a methylcarbonate.
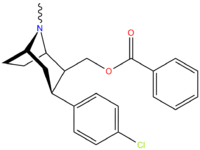
| Code | X | 2 Position | config | 8 | DA | 5-HT | NE |
| RTI-100 | F | -CH2OH | β,β | NMe | 47 | 4741 | no data |
| RTI-101 | I | -CH2OH | β,β | NMe | 2.2 | 26 | no data |
| RTI-99 | Br | -CH2OH | β,β | NMe | 1.49 | 51 | no data |
| RTI-93 | Cl | -CH2OH | β,β | NMe | 1.53 | 204 | 43.8 |
| RTI-105 | Cl | -CH2OAc | β,β | NMe | 1.60 | 143 | 127 |
| RTI-123 | Cl | -CH2OBz | β,β | NMe | 1.78 | 3.53 | 393 |
| RTI-145 | Cl | -CH2OCO2Me | β,β | NMe | 9.60 | 2.93 | 1.48 |
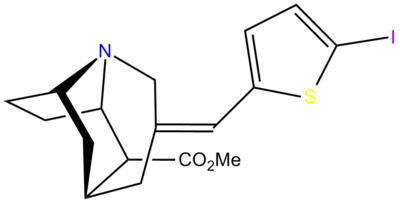
-ene-2%CE%B2-carbomethoxy-3%CE%B2-(4%E2%80%B2-chlorophenyl)tropane.png)
N-3-iodoprop-(2E)-ene-2β-carbomethoxy-3β-(4′-chlorophenyl)tropane.
A common radio-labeling research compound used as a ligand to map MAT.
β,α Stereochemistry
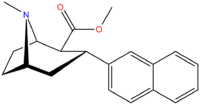
| Compound | X | 2 Group | config | 8 | DA | 5-HT | NE |
| RTI-140 | H | CO2Me | β,α | NMe | 101 | 5,701 | 2,076 |
| RTI-352 U.S. Patent 6,358,492 | I | CO2Me | β,α | NMe | 2.86 | 64.9 | 52.4 |
| RTI-549 | Br | CO2Me | β,α | NMe | — | — | — |
| RTI-319 U.S. Patent 7,011,813 | BN | CO2Me | β,α | NMe | 1.1 | 11.4 | 70.2 |
| RTI-286 U.S. Patent 7,011,813 | F | CO2Me | β,α | NMe | 21 | 5062 | 1231 |
| RTI-274 U.S. Patent 7,291,737 | F | CH2O(3′,4′-MD-phenyl) | β,α | NH | 3.96 | 5.62 | 14.4 |
| RTI-287 | Et | CO2Me | β,α | NMe | 327 | 1687 | 17,819 |
α,β Stereochemistry
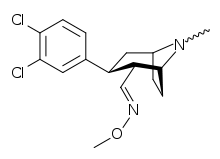
| Compound | DA (μM) | M.E.D. (mg/kg) | Dose(mg/kg) | Activity | Activity |
| (2R,3S)-2-(4-chlorophenoxymethyl)-8-methyl-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane | 0.39 | <1 | 50 | 0 | 0 |
| (2R,3S)-2-(carboxymethyl)-8-methyl-3-(2-naphthyl)-8-azabicyclo[3.2.1]octane | 0.1 | 1 | 25 | 0 | 0 |
| (2R,3S)-2-(carboxymethyl)-8-methyl-3-(3,4-dichlorophenyl)-8-azabicyclo[3.2.1]octane | 0.016 | 0.25 | 50 | + | +++ |
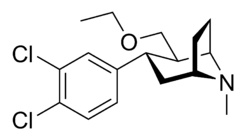
| Compound | X | 2 Group | config | 8 | DA | 5-HT | NE |
| Brasofensine | Cl2 | methyl aldoxime | α,β | NMe | — | — | — |
| Tesofensine | Cl2 | ethoxymethyl | α,β | NMe | 65 | 11 | 1.7 |
| NS-2359 (GSK-372,475) | Cl2 | Methoxymethyl | α,β | NH | — | — | — |
| Test Compound | DA uptake IC50(μM) | NA uptake IC50(μM) | 5-HT uptake IC50(μM) |
|---|---|---|---|
| (2R,3S)-2-(2,3-dichlorophenoxymethyl)-8-methyl-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane fumaric acid salt | 0.062 | 0.035 | 0.00072 |
| (2R,3S)-2-(Naphthaleneoxymethane)-8-methyl-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane fumaric acid salt | 0.062 | 0.15 | 0.0063 |
| (2R,3S)-2-(2,3-dichlorophenoxymethyl)-8-H-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane fumaric acid salt | 0.10 | 0.048 | 0.0062 |
| (2R,3S)-2-(Naphthlyloxymethane)-8-H-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane fumaric acid salt | 0.088 | 0.051 | 0.013 |
Aryl-Tropenes
| Test compound | DA-uptake IC50(μM) | NA-uptake IC50(μM) | 5-HT-uptake IC50(μM) |
| (+)-3-(4-Chlorophenyl)-8-H-aza-bicyclo[3.2.1]oct-2-ene | 0.26 | 0.028 | 0.010 |
| (+)-3-Napthalen-2-yl-8-azabicyclo[3.2.1]oct-2-ene | 0.058 | 0.013 | 0.00034 |
| (–)-8-Methyl-3-(naphthalen-2-yl)-8-azabicylo[3.2.1]oct-2-ene | 0.034 | 0.018 | 0.00023 |
| Test Compound | DA uptake IC50(μM) | NA uptake IC50(μM) | 5-HT uptake IC50(μM) |
|---|---|---|---|
| (±)-3-(3,4-Dichlorophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene | 0.079 | 0.026 | 0.0047 |
| Test Compound | DA uptake IC50(μM) | NA uptake IC50(μM) | 5-HT uptake IC50(μM) |
|---|---|---|---|
| (±)-3-(4-cyanophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene | 18 | 4.9 | 0.047 |
| (±)-3-(4-nitrophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene | 1.5 | 0.5 | 0.016 |
| (±)-3-(4-trifluoromethoxyphenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene | 22.00 | 8.00 | 0.0036 |
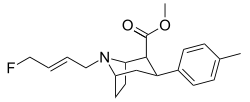
Heterocycles
These heterocycles are sometimes referred to as the "bioisosteric equivalent" of the simpler esters from which they are derived. A potential disadvantage of leaving the ββ-ester unreacted is that in addition to being hydrolyzable, it can also epimerize[7] to the energetically more favorable trans configuration. This can happen to cocaine also.
3-Substituted-isoxazol-5-yl
| Code | X | R | DA | NE | 5HT |
|---|---|---|---|---|---|
| RTI-165 | Cl | 3-methylisoxazol-5-yl | 0.59 | 181 | 572 |
| RTI-171 | Me | 3-methylisoxazol-5-yl | 0.93 | 254 | 3818 |
| RTI-180 | I | 3-methylisoxazol-5-yl | 0.73 | 67.9 | 36.4 |
| RTI-177 | Cl | 3-phenylisoxazol-5-yl | 1.28 | 504 | 2418 |
| RTI-176 | Me | 3-phenylisoxazol-5-yl | 1.58 | 398 | 5110 |
| RTI-181 | I | 3-phenylisoxazol-5-yl | 2.57 | 868 | 100 |
| RTI-184 | H | methyl | 43.3 | — | 6208 |
| RTI-185 | H | Ph | 285 | — | >12K |
| RTI-334 | Cl | 3-ethylisoxazol-5-yl | 0.50 | 120 | 3086 |
| RTI-335 | Cl | isopropyl | 1.19 | 954 | 2318 |
| RTI-336 | Cl | 3-(4-methylphenyl)isoxazol-5-yl | 4.09 | 1714 | 5741 |
| RTI-337 | Cl | 3-t-butyl-isoxazol-5-yl | 7.31 | 6321 | 37K |
| RTI-345 | Cl | p-chlorophenyl | 6.42 | 5290 | >76K |
| RTI-346 | Cl | p-anisyl | 1.57 | 762 | 5880 |
| RTI-347 | Cl | p-fluorophenyl | 1.86 | 918 | 7257 |
| RTI-354 | Me | 3-ethylisoxazol-5-yl | 1.62 | 299 | 6400 |
| RTI-366 | Me | R = isopropyl | 4.5 | 2523 (1550) | 42,900 (3900) |
| RTI-371 | Me | p-chlorophenyl | 8.74 | >100K (60,200) | >100K (9090) |
| RTI-386 | Me | p-anisyl | 3.93 | 756 (450) | 4027 (380) |
| RTI-387 | Me | p-fluorophenyl | 6.45 | 917 (546) | >100K (9400) |
3-Substituted-1,2,4-oxadiazole
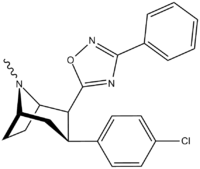
| Code | X | R | DA | NE | 5HT |
|---|---|---|---|---|---|
| ααRTI-87 | H | 3-methyl-1,2,4-oxadiazole | 204 | 36K | 30K |
| βαRTI-119 | H | 3-methyl-1,2,4-oxadiazole | 167 | 7K | 41K |
| αβRTI-124 | H | 3-methyl-1,2,4-oxadiazole | 1028 | 71K | 33K |
| RTI-125 | Cl | 3-methyl-1,2,4-oxadiazole | 4.05 | 363 | 2584 |
| ββRTI-126[8] | H | 3-methyl-1,2,4-oxadiazole | 100 | 7876 | 3824 |
| RTI-130 | Cl | 3-phenyl-1,2,4-oxadiazole | 1.62 | 245 | 195 |
| RTI-141 | Cl | 3-(p-anisyl)-1,2,4-oxadiazole | 1.81 | 835 | 357 |
| RTI-143 | Cl | 3-(p-chlorophenyl)-1,2,4-oxadiazole | 4.1 | 4069 | 404 |
| RTI-144 | Cl | 3-(p-bromophenyl)-1,2,4-oxadiazole | 3.44 | 1825 | 106 |
| βRTI-151 | Me | 3-phenyl-1,2,4-oxadiazole | 2.33 | 60 | 1074 |
| αRTI-152 | Me | 3-phenyl-1,2,4-oxadiazole | 494 | — | 1995 |
| RTI-154 | Cl | 3-isopropyl-1,2,4-oxadiazole | 6 | 135 | 3460 |
| RTI-155 | Cl | 3-cyclopropyl-1,2,4-oxadiazole | 3.41 | 177 | 4362 |
| Code | X | 2 Group | DA | NE | 5HT |
|---|---|---|---|---|---|
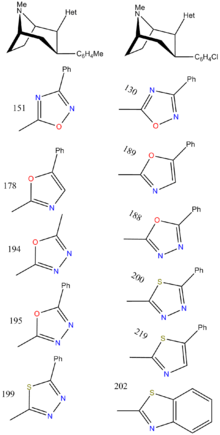
| RTI-157 | Me | tetrazole | 1557 | >37K | >43K |
| RTI-163 | Cl | tetrazole | 911 | — | 5456 |
| RTI-178 | Me | 5-phenyl-oxazol-2-yl | 35.4 | 677 | 1699 |
| RTI-188 | Cl | 5-phenyl-1,3,4-oxadiazol-2-yl | 12.6 | 930 | 3304 |
| RTI-189 | Cl | 5-phenyl-oxazol-2-yl | 19.7 | 496 | 1116 |
| RTI-194 | Me | 5-methyl-1,3,4-oxadiazol-2-yl | 4.45 | 253 | 4885 |
| RTI-195 | Me | 5-phenyl-1,3,4-oxadiazol-2-yl | 47.5 | 1310 | >22,000 |
| RTI-199 | Me | 5-phenyl-1,3,4-thiadiazol-2-yl | 35.9 | >24,000 | >51,000 |
| RTI-200 | Cl | 5-phenyl-1,3,4-thiadiazol-2-yl | 15.3 | 4142 | >18,000 |
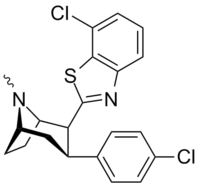
| RTI-202 | Cl | benzothiazol-2-yl | 1.37 | 403 | 1119 |
| RTI-219 | Cl | 5-phenylthiazol-2-yl | 5.71 | 8516 | 10,342 |
| RTI-262 | Cl | ||||
| RTI-370 | Me | 3-(p-cresyl)isoxazol-5-yl | 8.74 | 6980 | >100K |
| RTI-371 | Cl | 3-(p-chlorophenyl)isoxazol-5-yl | 13 | >100K | >100K |
| RTI-436 | Me | -CH=CHPh[10] | 3.09 | 1960 (1181) | 335 (31) |
| RTI-470 | Cl | o-Cl-benzothiazol-2-yl | 0.094 | 1590 (994) | 1080 (98) |
| RTI-451 | Me | benzothiazol-2-yl | 1.53 | 476 (287) | 7120 (647) |
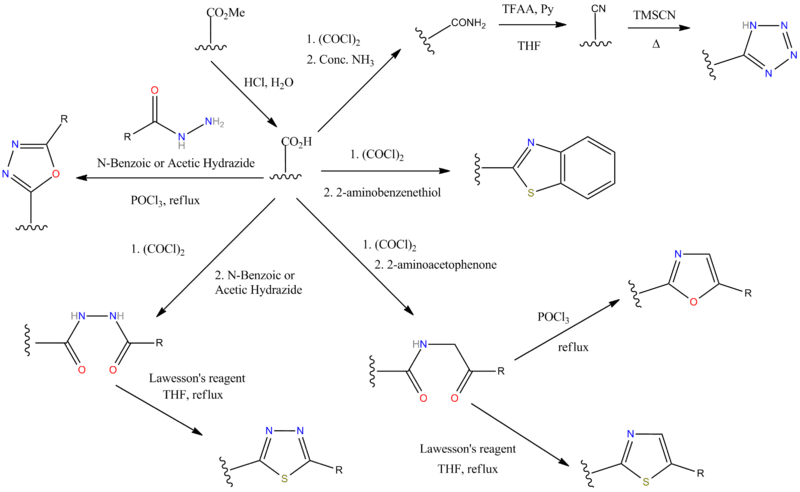
N.B There are some alternative ways of making the tetrazole ring though. C.f. the sartan drugs synthesis schemes. Bu3SnN3 is a milder choice of reagent than hydrogen azide (c.f. Irbesartan).
N-constrained
N-alkyl
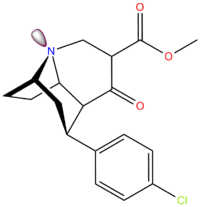
| Compound | X | 2 Group | config | 8 | DAT | SERT | NET |
| FP-β-CPPIT | Cl | 3′-phenylisoxazol-5′-yl | β,β | NCH2CH2CH2F | - | - | - |
| FE-β-CPPIT | Cl | (3′-phenylisoxazol-5′-yl) | β,β | NCH2CH2F | - | - | - |
| Altropane | F | CO2Me | β,β | NCH2CH=CHF | - | - | - |
| RTI-310 U.S. Patent 5,736,123 | I | CO2Me | β,β | N-Prn | 1.17 | - | - |
| RTI-311 | I | CO2Me | β,β | NCH2CH=CH2 | 1.79 | - | - |
| RTI-312 U.S. Patent 5,736,123 | I | CO2Me | β,β | NBun | 0.76 | - | - |
| RTI-313 U.S. Patent 5,736,123 | I | CO2Me | β,β | NCH2CH2CH2F | 1.67 | - | - |
| Ioflupane | ¹²³I | CO2Me | β,β | NCH2CH2CH2F | - | - | - |
| RTI-251 | Cl | CO2Me | β,β | NCH2CO2Et | 1.93 | 10.1 | 114 |
| RTI-252 | Cl | CO2Me | β,β | NCH2CH2CO2Et | 2.56 | 35.2 | 125 |
| RTI-242 | Cl | β,β (bridged) -C(O)CH(CO2Me)CH2N | 7.67 | 227 | 510 | ||
Bi- and tri-cyclic aza compounds and their uses U.S. Patent 6,150,376 WO 0007994
Tricyclic Tropanes
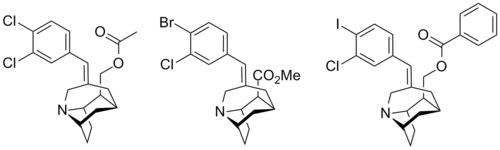
| Compound | X | Y | R | SERT Ki (nM) | DAT Ki (nM) | NET Ki (nM) |
| 1 | Cl | Cl | CH2OCOMe | 1.6 | 1870 | 638 |
| 2 | Br | Cl | CO2Me | 2.3 | 5420 | 459 |
| 3 | I | Cl | CH2OCOPh | 0.06 | >10K | >10K |
Kozikowski Fused
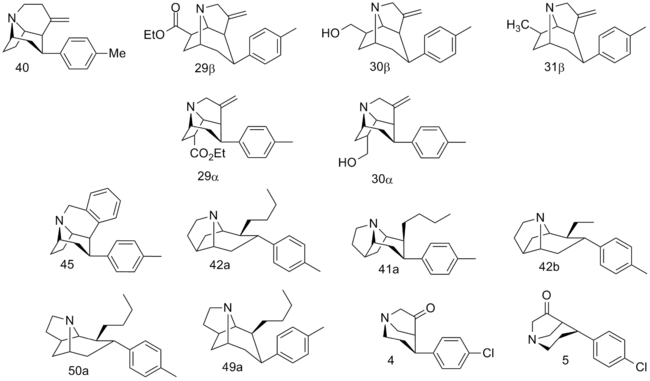
| Compound | Mazindol | DA | 5-HT | NE |
|---|---|---|---|---|
| cocaine | 375 | 423 | 155 | 83.3 |
| (–)-40 | 54.3 | 60.3 | 1.76 | 5.24 |
| (+)-40 | 79 | 114 | 1.48 | 4.62 |
| (±)40 | 61.7 | 60.3 | 2.32 | 2.69 |
| 29β | 620 | 1420 | 8030 | — |
| 30β | 186 | 492 | 97.7 | — |
| 31β | 47.0 | 211 | 28.5 | — |
| 29α | 4140 | 20100 | 3920 | — |
| 30α | 3960 | 8850 | 696 | 1150 |
| 45 | 6.86 | 24.0 | 1.77 | 1.06 |
| 42a | 4.00 | 2.23 | 14.0 | 2.99 |
| 41a | 17.2 | 10.2 | 78.9 | 15.0 |
| 42b | 3.61 | 11.3 | 25.7 | 4.43 |
| 50a | 149 | 149 | 810 | 51.7 |
| 49a | 13.7 | 14.2 | 618 | 3.84 |
| (–)-4 | 10500 | 16500 | 1890 | 70900 |
| (+)-4 | 18500 | 27600 | 4630 | 38300 |
| (–)-5 | 9740 | 9050 | 11900 | 4650 |
| (+)-5 | 6770 | 10500 | 25100 | 4530 |
Fused
Fused tropane-derivatives as neurotransmitter reuptake inhibitors. U.S. Patent 5,998,405
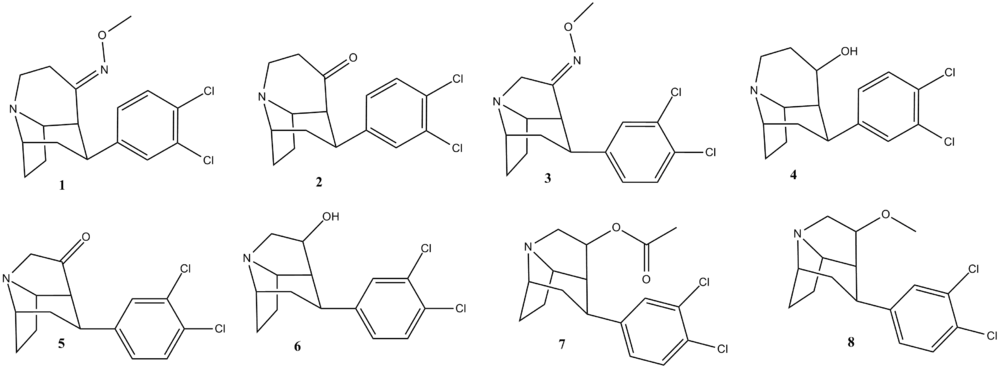
| Code | Compound | DA (μM) | NA (μM) | (μM)5-HT |
| 1 | (1 S,2S,4S,7R)-2-(3,4-Dichloro- phenyl)-8-azatricyclo[5.4.0.04,8]- undecan-11 -one O-methyl-oxime | 0.012 | 0.0020 | 0.0033 |
| 2 | (1 S,2S,4S,7R)-2-(3,4-Dichloro- phenyl)-8-azatricyclo[5.4.0.04,8]- undecan-11-one | 0.18 | 0.035 | 0.0075 |
| 3 | (1 S,3S,4S,8R)-3-(3,4-Dichloro-phenyl)-7-azatricyclo[5.3.0.04,8]- decan-5-one O-methyl-oxime | 0.0160 | 0.0009 | 0.0032 |
| 4 | (1 S,2S,4S,7R)-2-(3,4-Dichloro-phenyl)-8-azatricyclo[5.4.0.04,8]- undecan-11-ol | 0.0750 | 0.0041 | 0.0028 |
| 5 | (1 S,3S,4S,8R)-3-(3,4-Dichloro-phenyl)-7-azatricyclo[5.3.0.04,8]- decan-5-one | 0.12 | 0.0052 | 0.0026 |
| 6 | (1 S,3S,4S,8R)-3-(3,4-Dichloro- phenyl)-7-azatricyclo[5.3.0.04,8]-decan-5-ol | 0.25 | 0.0074 | 0.0018 |
| 7 | (1S,3S,4S,8R)-3- (3,4-Dichloro- phenyl)-7-azatricyclo[5.3.0.04,8]dec- 5-yl acetate | 0.21 | 0.0061 | 0.0075 |
| 8 | (1S,3S,4S,8R)-3-(3,4-Dichlorophenyl)-5-methoxy-7- azatricyclo[5.3.0.04,8]decane | 0.022 | 0.0014 | 0.0001 |
- 1-Chloroethyl chloroformate is used to remove N-methyl of trans-aryltropanes.
- 2° amine is reacted with Br(CH2)nCO2Et.
- Base used to abstract proton α- to CO2Et group and complete the tricyclic ring closure step (Dieckmann cyclization).
To make a different type of analog (see Kozikowski patent above)
- Remove N-Me
- Add ɣ-bromo-chloropropane
- Allow for cyclization with K2CO3 base and KI cat.
3-(2-thiophene) and 3-(2-furan)
| Code | Compound | DA (μM) | NA (μM) | (μM)5-HT |
| 1 | (2R,3S)-2-(2,3-Dichlorophenoxymethyl)-8-methyl-3-(2-thienyl)-8-aza-bicyclo[3.2.1]octanefumaric acid salt | 0.30 | 0.0019 | 0.00052 |
| 2 | (2R,3S)-2-(1-Naphthyloxymethyl)-8-methyl-3-(2-thienyl)-8-aza-bicyclo-[3.2.1]octane fumaric acid salt | 0.36 | 0.0036 | 0.00042 |
| 3 | (2R,3S)-2-(2,3-Dichlorophenoxymethyl)-8-methyl-3-(2-furanyl)-8-aza-bicyclo-[3.2.1]octane fumaric acid salt | 0.31 | 0.00090 | 0.00036 |
| 4 | (2R,3S)-2-(1-Naphthyloxymethyl)-8-methyl-3-(2-furanyl)-8-aza-bicyclo-[3.2.1]octane fumaric acid salt | 0.92 | 0.0030 | 0.00053 |
| 5 | (2R,3S)-2-(2,3-Dichlorophenoxymethyl)-8-H-3-(2-thienyl)-8-aza-bicyclo[3.2.1]octane fumaric acid salt | 0.074 | 0.0018 | 0.00074 |
| 6 | (2R,3S)-2-(1-Naphthyloxymethyl)-8-H-3-(2-thienyl)-8-aza-bicyclo[3.2.1]octane fumaric acid salt | 0.19 | 0.0016 | 0.00054 |
N-replaced (S,O,C)
| Compound | X | 2 Group | config | 8 | DA | 5-HT | NE |
| Tropoxane | Cl,Cl | CO2Me | (racemic) β,β | O | 3.3 | 6.5 | No data |
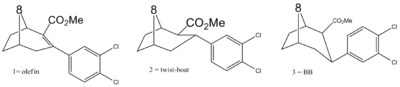
Diaryl
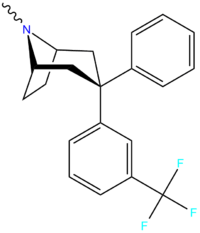 Hanna et al. (2007)[14] | 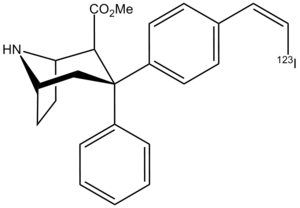 ZIENT:[15] |
Radiolabel Tropane
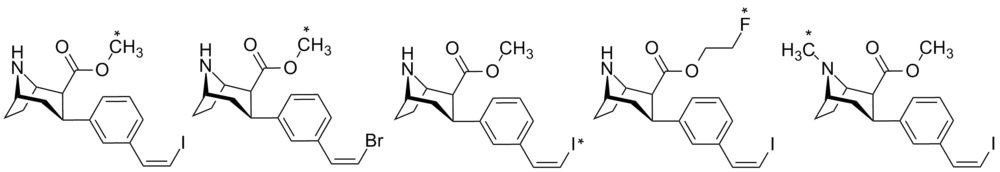
| Code | SERT Ki (nM) | NET Ki (nM) | DAT Ki (nM) | Radiolabel | In vivo study | Refs. |
| 1 | 0.2 | 102.2 | 29.9 | 11C | Non-human primate | [17] |
| 2 | 0.2 | 31.7 | 32.6 | 11C | Non-human primate | [18] |
| 3 | 0.05 | 24 | 3.47 | 123I | Rat | [19] |
| 4 | 0.08 | 28 | 13 | 18F | Non-human primate | [20] |
| 5 | 0.11 | 450 | 22 | 11C | Rat, monkey | [21] |
Irreversible
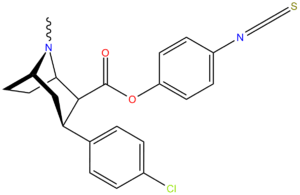
Irreversible (phenylisothiocyanate) binding ligand (Murthy, V.; Martin, T. J.; Kim, S.; Davies, H. M. L.; Childers, S. R. (2008). "In Vivo Characterization of a Novel Phenylisothiocyanate Tropane Analog at Monoamine Transporters in Rat Brain". Journal of Pharmacology and Experimental Therapeutics 326 (2): 587–595. doi:10.1124/jpet.108.138842. PMID 18492949.)[22] RTI-76:[23] 4′-isothiocyanatophenyl (1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate. Also known as: 3β-(p-chlorophenyl)tropan-2β-carboxylic acid p-isothiocyanatophenylmethyl ester.
Note the contrast to the phenylisothiocyanate covalent binding site location as compared to the one on p-Isococ, a non-phenyltropane cocaine analogue.
Nortropanes (N-demethylated)
NS2359 (GSK-372,475)
It is well established that electrostatic potential around the para position tends to improve MAT binding. This is believed to also be the case for the meta position, although it is less studied. N-demethylation dramatically potentiates NET and SERT affinity, but the effects of this on DAT binding are insignificant.[24] Of course, this is not always the case. For an interesting exception to this trend, see the Taxil document. There is ample evidence suggesting that N-demethylation of alkaloids occurs naturally in vivo via a biological enzyme. The fact that hydrolysis of the ester leads to inactive metabolites means that this is still the main mode of deactivation for analogues that have an easily metabolised 2-ester substituent. The attached table provides good illustration of the effect of this chemical transformation on MAT binding affinities. N.B. In the case of both nocaine and pethidine, N-demethyl compounds are more toxic and have a decreased seizure threshold.[25]
| Code | X | DA | 5HT | NE |
|---|---|---|---|---|
| RTI-142 | F | 4.39 | 68.6 | 18.8 |
| RTI-98 | I | 0.69 | 0.36 | 11.0 |
| RTI-110 | Cl | 0.62 | 4.13 | 5.45 |
| RTI-173 | Et | 49.9 | 8.13 | 122 |
| X | [3H]Paroxetine | [3H]WIN 35,428 | [3H]Nisoxetine |
|---|---|---|---|
| Ethyl | 28.4 → 8.13 | 55 → 49.9 | 4,029 → 122 |
| vinyl | 9.5 → 2.25 | 1.24 → 1.73 | 78 → 14.9 |
| Ethynyl | 4.4 → 1.59 | 1.2 → 1.24 | 83.2 → 21.8 |
| 1-Propyl | 70.4 → 26 | 68.5 → 212 | 3,920 → 532 |
| trans-propenyl | 11.4 → 1.3 | 5.29 → 28.6 | 1,590 → 54 |
| cis-propenyl | 7.09 → 1.15 | 15 → 31.6 | 2,800 → 147 |
| Allyl | 28.4 → 6.2 | 32.8 → 56.5 | 2,480 → 89.7 |
| 1-Propynyl | 15.7 → 3.16 | 2.37 → 6.11 | 820 → 116 |
| i-Propyl | 191 → 15.1 | 597 → 310 | 75,000 → ? |
| 2-Propenyl | 3.13 → 0.6 | 14.4 → 23 | 1,330? → 144 |
| Isomer | 4′ | 3′ | NE | DA | 5HT |
|---|---|---|---|---|---|
| β,β | Me | H | 60 → 7.2 | 1.7 → 0.84 | 240 → 135 |
| β,β | F | H | 835 → 18.8 | 15.7 → 4.4 | 760 → 68.6 |
| β,β | Cl | H | 37 → 5.45 | 1.12 → 0.62 | 45 → 4.13 |
| β,α | Me | H | 270 → 9 | 10.2 → 33.6 | 4250 → 500 |
| β,α | F | H | 1200 → 9.8 | 21 → 32.6 | 5060 → 92.4 |
| β,α | Cl | H | 60 → 5.41 | 2.4 → 3.1 | 998 → 53.3 |
| β,α | F | Me | 148 → 4.23 | 13.7 → 9.38 | 1161 → 69.8 |
| β,α | Me | F | 44.7 → 0.86 | 7.38 → 9 | 1150 → 97.4 |
"Interest in NET selective drugs continues as evidenced by the development of atomoxetine, manifaxine, and reboxetine as new NET selective compounds for treating ADHD and other CNS disorders such as depression" (FIC, et al. 2005).[26]
Thiophenyltropanes
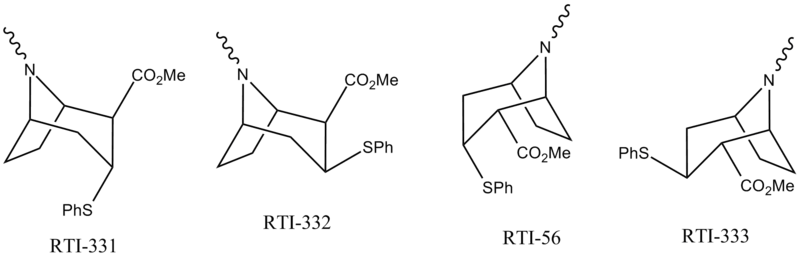
Select annotations of above
Phenyltropanes can be grouped by "N substitution" "Stereochemistry" "2-substitution" & by the nature of the 3-phenyl group substituent X.
Often this has dramatic effects on selectivity, potency, and duration, also toxicity, since phenyltropanes are highly versatile. For more examples of interesting phenyltropanes, see some of the more recent patents, e.g. U.S. Patent 6,329,520, U.S. Patent 7,011,813, U.S. Patent 6,531,483, and U.S. Patent 7,291,737.
Potency in vitro should not be confused with the actual dosage, as pharmacokinetic factors can have a dramatic influence on what proportion of an administered dose actually gets to the target binding sites in the brain, and so a drug that is very potent at binding to the target may nevertheless have only moderate potency in vivo. For example, RTI-336 requires a higher dosage than cocaine. Accordingly, the active dosage of RTI-386 is exceedingly poor despite the relatively high ex vivo DAT binding affinity.
Sister substances
Many molecular drug structures have exceedingly similar pharmarcology to phenyltropanes, yet by certain technicalities do not fit the phenyltropane moniker. These are namely classes of dopaminergic cocaine analogues that are in the piperidine class (a category that includes methylphenidate) or benztropine class (such as Difluoropine: which is extremely close to fitting the criteria of being a phenyltropane.) Whereas other potent DRIs are far removed from being in the phenyltropane structural family, such as Benocyclidine or Vanoxerine.
Misc.
| Code | X | 2 Position | config | 8 | DA | 5-HT | NE |
| RTI-102 | I | CO2H | β,β | NMe | 474 | 1928 | 43,400 |
| RTI-103 | Br | CO2H | β,β | NMe | 278 | 3070 | 17,400 |
| RTI-104 | F | CO2H | β,β | NMe | 2744 | >100K | >100K |
| RTI-108 | Cl | -CH2Cl | β,β | NMe | 2.64 | 98 | 129.8 |
| RTI-241 | Me | -CH2CO2Me | β,β | NMe | 1.02 | 619 | 124 |
| RTI-139 | Cl | -CH3 | β,β | NMe | 1.67 | 85 | 57 |
| RTI-161 | Cl | -C≡N | β,β | NMe | 13.1 | 1887 | 2516 |
| RTI-230 | Cl | H3C–C=CH2 | β,β | NMe | 1.28 | 57 | 141 |
| RTI-240 | Cl | -CHMe2 | β,β | NMe | 1.38 | 38.4 | 84.5 |
| RTI-145 | Cl | -CH2OCO2Me | β,β | NMe | 9.60 | 2,932 | 1,478 |
| RTI-158 | Me | -C≡N | β,β | NMe | 57 | 5095 | 1624 |
| RTI-131 | Me | -CH2NH2 | β,β | NMe | 10.5 | 855 | 120 |
| RTI-164 | Me | -CH2NHMe | β,β | NMe | 13.6 | 2246 | 280 |
| RTI-132 | Me | -CH2NMe2 | β,β | NMe | 3.48 | 206 | 137 |
| RTI-239 | Me | -CHMe2 | β,β | NMe | 0.61 | 114 | 35.6 |
| RTI-338 | Et | -CO2CH2Ph | β,β | NMe | 1104 | 7.41 | 3366 |
| RTI-348 | H | -Ph | β,β | NMe | 28.2 | >34,000 | 2670 |
Phenyltropane based Benztropines
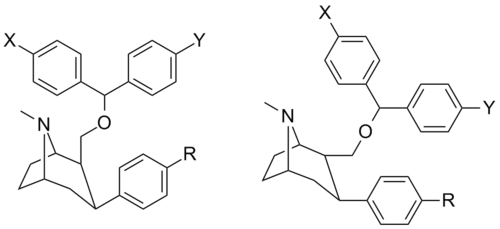
F&B series (Biotin side-chains etc.)
The compound of the present invention are useful pesticides.[8]
| Code | X | 2 Position | config | DA | NE | 5-HT |
| RTI-224 | Me | F1c | β,β | 4.49 | — | 155.6 |
| RTI-233 | Me | F2 | β,β | 4.38 | 516 | 73.6 |
| RTI-235 | Me | F3 d | β,β | 1.75 | 402 | 72.4 |
| RTI-236 | Me | B1 d | β,β | 1.63 | 86.8 | 138 |
| RTI-237 | Me | B2 d | β,β | 7.27 | 258 | 363 |
| RTI-244 | Me | B3 d | β,β | 15.6 | 1809 | 33.7 |
| RTI-245 | Cl | F4 c | β,β | 77.3 | — | — |
| RTI-246 | Me | F4 c | β,β | 50.3 | 3000 | — |
| RTI-248 | Cl | F6 c | β,β | 9.73 | 4674 | 6.96 |
| RTI-249 | Cl | F1 c | β,β | 8.32 | 5023 | 81.6 |
| RTI-266 | Me | F2 | β,β | 4.80 | 836 | 842 |
| RTI-267 | Me | F7 wrong | β,β | 2.52 | 324 | 455 |
| RTI-268 | Me | F7 right | β,β | 3.89 | 1014 | 382 |
| RTI-269 | Me | F8 | β,β | 5.55 | 788 | 986 |
See also
References
- ↑ 1.0 1.1 Tamagnan, Gilles (2005). "Synthesis and monoamine transporter affinity of new 2β-carbomethoxy-3β-[4-(substituted thiophenyl)]phenyltropanes: discovery of a selective SERT antagonist with picomolar potency". Bioorganic 15 (4): 1131–1133. doi:10.1016/j.bmcl.2004.12.014. PMID 15686927.
- ↑ U.S. Patent 6,479,509 Method of promoting smoking cessation.
- ↑ Blough, B. E.; Keverline, K. I.; Nie, Z.; Navarro, H.; Kuhar, M. J.; Carroll, F. I. (2002). "Synthesis and transporter binding properties of 3beta-4′-(phenylalkyl, -phenylalkenyl, and -phenylalkynyl)phenyltropane-2β-carboxylic acid methyl esters: evidence of a remote phenyl binding domain on the dopamine transporter". Journal of Medicinal Chemistry 45 (18): 4029–4037. doi:10.1021/jm020098n. PMID 12190324.
- ↑ 4.0 4.1 4.2 Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists. Satendra Singh et al. Chem. Rev. 2000, 100. 925-1024. PubMed; Chemical Reviews (Impact Factor: 45.66). 04/2000; 100(3):925-1024 American Chemical Society; 2000, ISSN: 0009-2665 ChemInform; May, 16th 2000, Volume 31, Issue 20, DOI: 10.1002/chin.200020238. Mirror hotlink.
- ↑ 5.0 5.1 Meltzer, P. C.; McPhee, M.; Madras, B. K. (2003). "Synthesis and biological activity of 2-Carbomethoxy-3-catechol-8-azabicyclo[3.2.1]octanes". Bioorganic & Medicinal Chemistry Letters 13 (22): 4133. doi:10.1016/j.bmcl.2003.07.014.
- ↑ http://www3.interscience.wiley.com/journal/55001898/abstract
- ↑ Carroll, F. I.; Gray; Abraham; Kuzemko; Lewin; Boja; Kuhar (1993). "3-Aryl-2-(3′-substituted-1′,2′,4'-oxadiazol-5′-yl)tropane analogues of cocaine: affinities at the cocaine binding site at the dopamine, serotonin, and norepinephrine transporters". Journal of Medicinal Chemistry 36 (20): 2886–2890. doi:10.1021/jm00072a007. PMID 8411004.
- ↑ 8.0 8.1 Methods for controlling invertebrate pests using cocaine receptor binding ligands. U.S. Patent 5,935,953
- ↑ Carroll, F.; Howard, J.; Howell, L.; Fox, B.; Kuhar, M. (2006). "Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse". The AAPS journal 8 (1): E196–E203. doi:10.1208/aapsj080124. PMC 2751440. PMID 16584128.
- ↑ Carroll, F.; Howard, J.; Howell, L.; Fox, B.; Kuhar, M. (2006). "Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse". The AAPS journal 8 (1): E196–E203. doi:10.1208/aapsj080124. PMC 2751440. PMID 16584128.
- ↑ Hoepping, Alexander (2000). "Novel Conformationally Constrained Tropane Analogues by 6- e ndo-trig Radical Cyclization and Stille Coupling − Switch of Activity toward the Serotonin and/or Norepinephrine Transporter". Journal of Medicinal Chemistry 43 (10): 2064–2071. doi:10.1021/jm0001121.
- ↑ Zhang, Ao (2002). "Thiophene derivatives: a new series of potent norepinephrine and serotonin reuptake inhibitors". Bioorganic 12 (7): 993–995. doi:10.1016/S0960-894X(02)00103-8.
- ↑ Zhang, Ao (2002). "Further Studies on Conformationally Constrained Tricyclic Tropane Analogues and Their Uptake Inhibition at Monoamine Transporter Sites: Synthesis of ( Z )-9-(Substituted arylmethylene)-7-azatricyclo[4.3.1.0 3,7 ]decanes as a Novel Class of Serotonin Transporter Inhibitors". Journal of Medicinal Chemistry 45 (9): 1930–1941. doi:10.1021/jm0105373.
- ↑ Hanna, Mona M. (2007). "Synthesis of some tropane derivatives of anticipated activity on the reuptake of norepinephrine and/or serotonin". Bioorganic 15 (24): 7765–7772. doi:10.1016/j.bmc.2007.08.055.
- ↑ Goodman, Mark M. (2003). "Synthesis and Characterization of Iodine-123 Labeled 2β-Carbomethoxy-3β-(4′-(( Z )-2-iodoethenyl)phenyl)nortropane. A Ligand for in Vivo Imaging of Serotonin Transporters by Single-Photon-Emission Tomography". Journal of Medicinal Chemistry 46 (6): 925–935. doi:10.1021/jm0100180.
- ↑ "Transporters as Targets for Drugs". Topics in Medicinal Chemistry. 2009. doi:10.1007/978-3-540-87912-1.
- ↑ Stehouwer, Jeffrey S. (2006). "Synthesis, Radiosynthesis, and Biological Evaluation of Carbon-11 Labeled 2β-Carbomethoxy-3β-(3′-(( Z )-2-haloethenyl)phenyl)nortropanes: Candidate Radioligands for in Vivo Imaging of the Serotonin Transporter with Positron Emission Tomography". Journal of Medicinal Chemistry 49 (23): 6760–6767. doi:10.1021/jm060641q.
- ↑ Deskus, Jeffrey A. (2007). "Conformationally restricted homotryptamines 3. Indole tetrahydropyridines and cyclohexenylamines as selective serotonin reuptake inhibitors". Bioorganic 17 (11): 3099–3104. doi:10.1016/j.bmcl.2007.03.040.
- ↑ Schmitz, William D. (2005). "Homotryptamines as potent and selective serotonin reuptake inhibitors (SSRIs)". Bioorganic 15 (6): 1619–1621. doi:10.1016/j.bmcl.2005.01.059.
- ↑ Plisson, Christophe (2007). "Synthesis and in Vivo Evaluation of Fluorine-18 and Iodine-123 Labeled 2β-Carbo(2-fluoroethoxy)-3β-(4′-(( Z )-2-iodoethenyl)phenyl)nortropane as a Candidate Serotonin Transporter Imaging Agent". Journal of Medicinal Chemistry 50 (19): 4553–4560. doi:10.1021/jm061303s.
- ↑ PMC 2671940
- ↑ Carroll, F. I.; Gao; Abraham; Lewin; Lew; Patel; Boja; Kuhar (1992). "Probes for the cocaine receptor. Potentially irreversible ligands for the dopamine transporter". Journal of Medical Chemistry 35 (10): 1813–1817. doi:10.1021/jm00088a017. PMID 1588560.
- ↑ Wu; Reith, M.; Walker, Q.; Kuhn, C.; Carroll, F.; Garris, P. (2002). "Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study". Journal of Neuroscience 22 (14): 6272–6281. PMID 12122086.
- ↑ Blough, B.; Abraham, P.; Lewin, A.; Kuhar, M.; Boja, J.; Carroll, F. (1996). "Synthesis and transporter binding properties of 3β-(4′-alkyl-, 4′-alkenyl-, and 4′-alkynylphenyl)nortropane-2β-carboxylic acid methyl esters: serotonin transporter selective analogs". Journal of Medicinal Chemistry 39 (20): 4027–4035. doi:10.1021/jm960409s. PMID 8831768.
- ↑ Spealman, R. D.; Kelleher, R. T. (Mar 1981). "Self-administration of cocaine derivatives by squirrel monkeys". The Journal of Pharmacology and Experimental Therapeutics 216 (3): 532–536. ISSN 0022-3565. PMID 7205634.
- ↑ Carroll, F.; Tyagi, S.; Blough, B.; Kuhar, M.; Navarro, H. (2005). "Synthesis and monoamine transporter binding properties of 3alpha-(substituted phenyl)nortropane-2beta-carboxylic acid methyl esters. Norepinephrine transporter selective compounds". Journal of Medicinal Chemistry 48 (11): 3852–3857. doi:10.1021/jm058164j. PMID 15916437.
- ↑ Xu, L.; Kulkarni, S. S.; Izenwasser, S.; Katz, J. L.; Kopajtic, T.; Lomenzo, S. A.; Newman, A. H.; Trudell, M. L. (2004). "Synthesis and Monoamine Transporter Binding of 2-(Diarylmethoxymethyl)-3β-aryltropane Derivatives". Journal of Medicinal Chemistry 47 (7): 1676. doi:10.1021/jm030430a.
External links
- U. S. Provisional Patent Application listing examples of compounds that are tropanes for prospective use in research
- Article on cocaine analogue research
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||