List of methylphenidate analogues
This is a list of methylphenidate (MPH or MPD) analogues. Regular methylphenidate can come in several varieties. Including: the racemate, the enantiopure (dextro or levo) of its stereoisomers; erythro or threo (either + or -) among its diastereoisomers & lastly the isomers S,S; S,R/R,S or R,R. The variant with optimized efficacy is not the usually attested generic or common pharmaceutical brands (e.g. Ritalin, Daytrana etc.) but the (R,R)-dextro-(+)-threo. Which has a binding profile on par with or better than that of cocaine.[lower-alpha 1]
Also of note is that methylphenidate in demethylated form is acidic; a conformation known as ritalinic acid.[2] This gives the potential to yield a conjugate salt[3] form effectively protonated by a salt nearly chemically duplicate/identical to its own structure; creating a "methylphenidate ritalinate".[4]
The carboxymethyl (methyl acetate) has sometimes been replaced with similar length ketones to increase duration. For instance, the methoxycarbonyl has had examples of having been replaced with an alkyl group (such as Kozikowski showed with RTI-31 n-propyl residue. c.f.[5])
Desoxypipradrol (and thus Pipradrol, including such derivatives as AL-1095, Diphemethoxidine, SCH-5472 & D2PM), and even mefloquine could be considered vaguely related structurally, with the former ones also functionally so, as loosely analogous compounds.
| Table of methylphenidate variants: click to | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | IC50 (nM) (Inhibition of [3H]WIN 35428 binding) |
IC50 (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|---|
 | ||||||
| (D-threo-methylphenidate) | H, H | 33 | 244 ± 142 (171 ± 10) | 7.4 | ||
| (L-threo-methylphenidate) | 540 | 5100 (1468 ± 112) | 9.4 | |||
| (D/L-threo-methylphenidate) "eudismic ratio" | 6.4 | 20.9 (8.6) | - | |||
| (DL-threo-methylphenidate) | 83.0 ± 7.9 | 224 ± 19 | 2.7 | |||
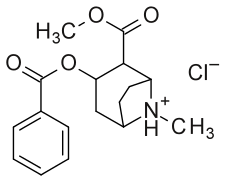 | (R-benzoyl-methylecgonine) (cocaine) | (H, H) | 173 ± 13 | 404 ± 26 | 2.3 | |
 | ||||||
| 351a | F | H y d r o g e n i.e. H | 35.0 ± 3.0 | 142 ± 2.0 | 4.1 | |
| 351b | Cl | 20.6 ± 3.4 | 73.8 ± 8.1 | 3.6 | ||
| 351c | Br | 6.9 ± 0.1 | 26.3 ± 5.8 | 3.8 | ||
| 351d | (d) Br | - | 22.5 ± 2.1 | - | ||
| 351e | (l) Br | - | 408 ± 17 | - | ||
| 351d/e "eudismic ratio" | (d/l) Br | - | 18.1 | - | ||
| 351f | I | 14.0 ± 0.1 | 64.5 ± 3.5 | 4.6 | ||
| 351g | OH | 98.0 ± 10 | 340 ± 70 | 3.5 | ||
| 351h | OCH3 | 83 ± 11 | 293 ± 48 | 3.5 | ||
| 351i | (d) OCH3 | - | 205 ± 10 | - | ||
| 351j | (l) OCH3 | - | 3588 ± 310 | - | ||
| 351i/j "eudismic ratio" | (d/l) OCH3 | - | 17.5 | - | ||
| 351k | CH3 | 33.0 ± 1.2 | 126 ± 1 | 3.8 | ||
| 351l | t-Bu | 13500 ± 450 | 9350 ± 950 | 0.7 | ||
| 351m | NH2.HCl | 34.6 ± 4.0 | 115 ± 10 | 3.3 | ||
| 351n | NO2 | 494 ± 33 | 1610 ± 210 | 3.3 | ||
 | ||||||
| 352a | F | 40.5 ± 4.5 | 160 ± 0.00 | 4.0 | ||
| 352b | Cl | 5.1 ± 1.6 | 23.0 ± 3.0 | 4.5 | ||
| 352c | Br | 4.2 ± 0.2 | 12.8 ± 0.20 | 3.1 | ||
| 352d | OH | 321 ± 1.0 | 790 ± 30 | 2.5 | ||
| 352e | OMe | 288 ± 53 | 635 ± 35 | 0.2 | ||
| 352f | Me | 21.4 ± 1.1 | 100 ± 18 | 4.7 | ||
| 352g | NH2.HCl | 265 ± 5 | 578 ± 160 | 2.2 | ||
 | 353a | F | 1420 ± 120 | 2900 ± 300 | 2.1 | |
| 353b | Cl | 1950 ± 230 | 2660 ± 140 | 1.4 | ||
| 353c | Br | 1870 ± 135 | 3410 ± 290 | 1.8 | ||
| 353d | OH | 23100 ± 50 | 35,800 ± 800 | 1.6 | ||
| 353e | OCH3 | 101,000 ± 10,000 | 81,000 ± 2000 | 0.8 | ||
 | 354a | Cl, Cl | 5.3 ± 0.7 | 7.0 ± 0.6 | 1.3 | |
| 354b | I | OH | 42 ± 21 | 195 ± 197 | 4.6 | |
| 354c | OMe, OMe | 810 ± 10 | 1760 ± 160 | 2.2 | ||
Both analogues 374 & 375 displayed higher potency than methylphenidate at DAT. In further comparison, 375 (the 2-naphthyl) was additionally two & a half times more potent than 374 (the 1-naphthyl isomer).[lower-alpha 3]
| Compound | S. Singh's alphanumeric assignation (name) |
Ring | Ki (nM) (Inhibition of [125I]IPT binding) |
Ki (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|
 | (D-threo-methylphenidate) | benzene | 324 | - | - |
 | (DL-threo-methylphenidate) | 82 ± 77 | 429 ± 88 | 0.7 | |
 | 374 | 1-naphthalene | 194 ± 15 | 1981 ± 443 | 10.2 |
 | 375 (HDMP-28) | 2-naphthalene | 79.5 | 85.2 ± 25 | 1.0 |
 | 376 | benzyl | >5000 | - | - |

| Compound | S. Singh's alphanumeric assignation (name) |
R | IC50 (nM) (Inhibition of binding at DAT) |
|---|---|---|---|
 | |||
| 373a | H | 500 ± 25 | |
| 373b | 4″-OH | 1220 ± 140 | |
| 373c | 4″-CH3 | 139 ± 13 | |
| 373d | 3″-Cl | 161 ± 18 | |
| 373e | 3″-Me | 108 ± 16 |
| Compound | S. Singh's alphanumeric assignation (name) |
Cycloalkane ring |
Ki (nM) (Inhibition of binding) |
|---|---|---|---|
 | 380 | 2-pyrrolidine (cyclopentane) | 1336 ± 108 |
 | 381 | 2-azepane (cycloheptane) | 1765 ± 113 |
 | 382 | 2-azocane (cyclooctane) | 3321 ± 551 |
 | 383 | 4-1,3-oxazinane (cyclohexane) | 6689 ± 1348 |

See also
External links
- "Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters." doi:10.1016/0024-3205(96)00052-5 Mirror hotlink
- "Azido-iodo-N-benzyl derivatives of threo-methylphenidate (Ritalin, Concerta): Rational design, synthesis, pharmacological evaluation, and dopamine transporter photoaffinity labeling." Bioorg Med Chem. 2011 Jan 1;19(1):504-12. doi: 10.1016/j.bmc.2010.11.002. Epub 2010 Nov 4.
- "Uses of methylphenidate derivatives" Google patents. Pub. # US 20060183773 A1
- Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter. J Med Chem. 2007 Jan 25;50(2):219-32.
- Synthesis of methylphenidate analogues and their binding affinities at dopamine and serotonin transport sites. doi:10.1016/j.bmcl.2003.12.097
- Vinylogous amide analogs of methylphenidate. Bioorg Med Chem Lett. 2005 Jun 15;15(12):3044-7.
- Biochemical and Behavioral Characterization of Novel Methylphenidate Analogs doi: 10.1124/jpet.301.2.527
- Methylphenidate analogs with behavioral differences interact differently with arginine residues on the dopamine transporter in rat striatum doi: 10.1002/syn.20161
- Quantitative structure-activity relationship studies of threo-methylphenidate analogs.
- Evolution of a Compact Photoprobe for the Dopamine Transporter Based on (±)-threo-Methylphenidate doi: 10.1021/ml3000098
- Synthesis and pharmacology of site specific cocaine abuse treatment agents: a new synthetic methodology for methylphenidate analogs based on the Blaise reaction European Journal of Medicinal Chemistry. Howard M Deutsch et al. Volume 36, Issue 4, April 2001, Pages 303–311
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists. Satendra Singh et al. Chem. Rev. 2000, 100. 925-1024. PubMed; Chemical Reviews (Impact Factor: 45.66). 04/2000; 100(3):925-1024 American Chemical Society; 2000, ISSN: 0009-2665 ChemInform; May, 16th 2000, Volume 31, Issue 20, DOI: 10.1002/chin.200020238. Mirror hotlink.
- ↑ Correlation between methylphenidate and ritalinic acid concentrations in oral fluid and plasma. Clin Chem. 2010 Apr;56(4):585-92. doi: 10.1373/clinchem.2009.138396. PMID 20167695
- ↑ Process for the preparation of dexmethylphenidate hydrochloride Google patents; Publication #US 20040180928 A1
- ↑ Resolution of ritalinic acid salt Google patents; Publication #US6441178 B2
- ↑ Froimowitz, M.; Gu, Y.; Dakin, L.; Nagafuji, P.; Kelley, C.; Parrish, D.; Deschamps, J.; Janowsky, A. (2007). "Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter". Journal of Medicinal Chemistry 50 (2): 219–232. doi:10.1021/jm0608614. PMID 17228864.
- ↑ Markowitz JS, Zhu HJ, Patrick KS (2013). "Isopropylphenidate: an ester homolog of methylphenidate with sustained and selective dopaminergic activity and reduced drug interaction liability". J Child Adolesc Psychopharmacol 23 (10): 648–54. doi:10.1089/cap.2013.0074. PMID 24261661.
- ↑ John S. Markowitz, Kennerly S. Patrick, Haojie Zhu (Sep 27, 2012). "Patent US20120245201 - Isopropylphenidate for Treatment of Attention-Deficit/Hyperactivity Disorder and Fatigue-Related Disorders and Conditions". Retrieved 15 August 2014.
- ↑ The Reinforcing Efficacy of Psychostimulants in Rhesus Monkeys: The Role of Pharmacokinetics and Pharmacodynamics 0022-3565/03/3071-356–366 The Journal Of Pharmacology And Experimental Therapeutics. Vol. 307, No. 1
- ↑ [1] ←Page #1,005 (81st page of article) §VI. Final ¶.
- ↑ [1] ←Page #1,010 (86th page of article) Table 47, Page #1,007 (83rd page of article) Figure 52
- ↑ [1] ←Page #1,010 (86th page of article) 2nd ¶, lines 2, 3 & 5.
- ↑ [1] ←Page #1,010 (86th page of article) Table 49, Page #1,007 (83rd page of article) Figure 54
- ↑ [1] ←Page #1,010 (86th page of article) Table 48, Page #1,007 (83rd page of article) Figure 53
- ↑ [1] ←Page #1,011 (87th page of article) Table 50, Page #1,007 (83rd page of article) Figure 55
| Wikimedia Commons has media related to Methylphenidate. |
| ||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||
| ||||||||||||||||||||||||||||||||||






