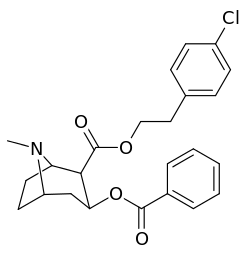
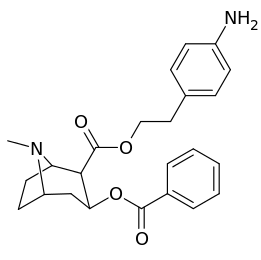
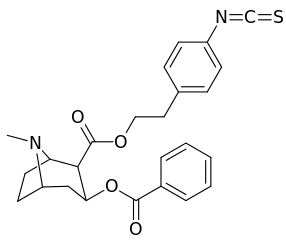
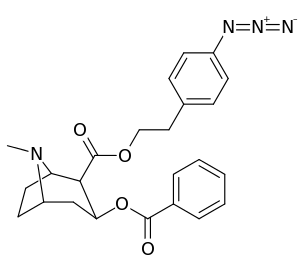
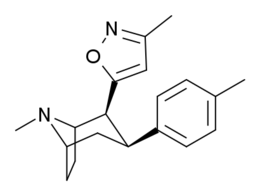
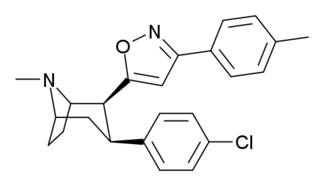
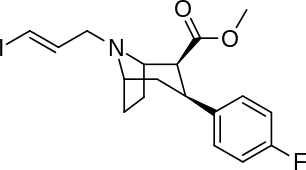
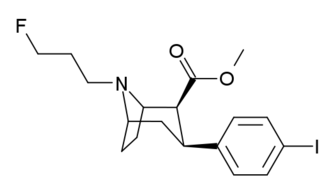
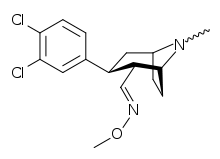
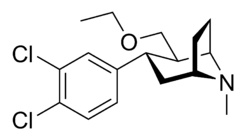
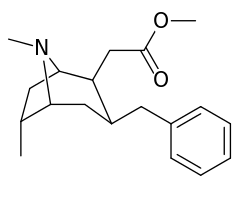
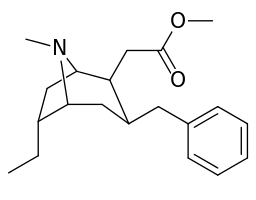
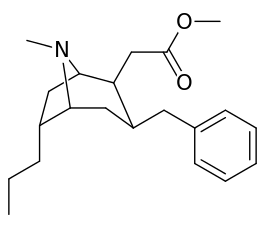
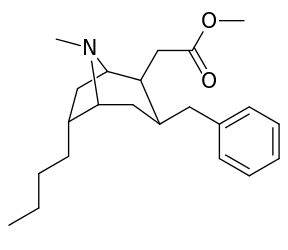
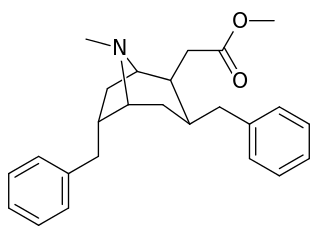
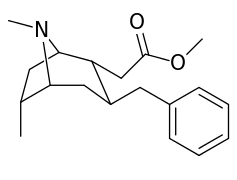
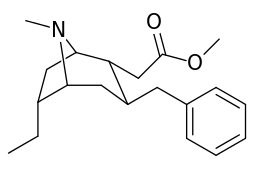
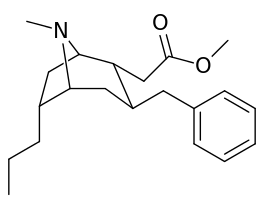
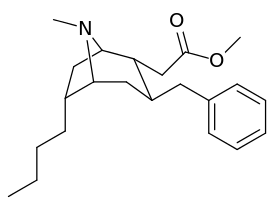
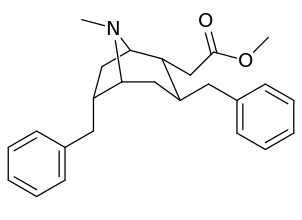
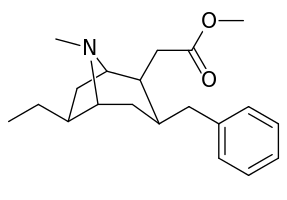
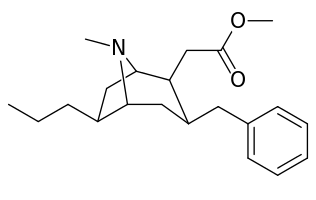
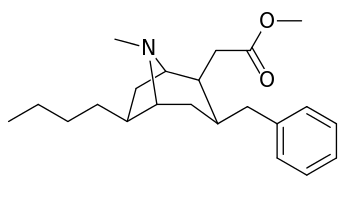
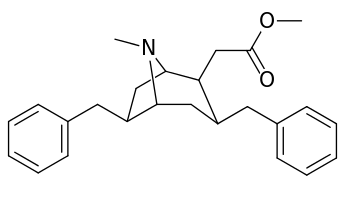
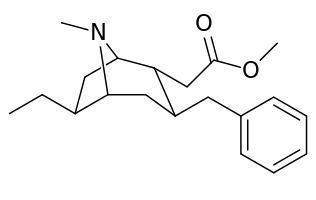
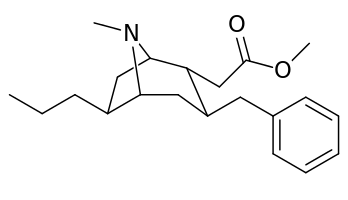
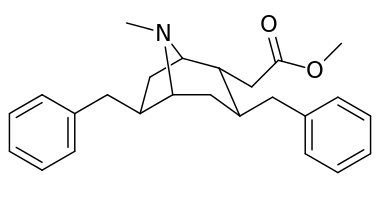
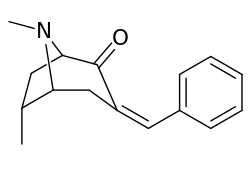
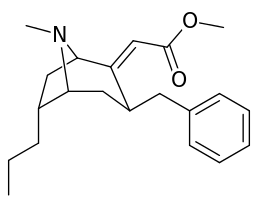
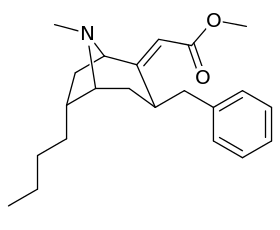
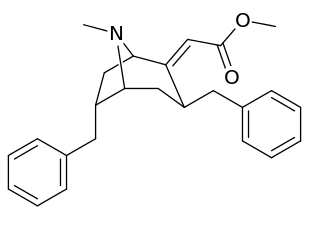
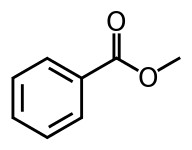
List of cocaine analogues
This is a list of cocaine analogues. A cocaine analogue retains 3β-benzoyloxy or similar functionality (the term specifically used usually distinguishes from phenyltropanes, but generally as a category includes them). Many of the semi-synthetic cocaine analogues proper which have been made & studied have consisted of among the nine following classes of compounds:[lower-alpha 1]
- stereoisomers of cocaine
- 3β-phenyl ring substituted analogues
- 2β-substituted analogues
- N-modified analogues of cocaine
- 3β-carbamoyl analogues
- 3β-alkyl-3-benzyl tropanes
- 6/7-substituted cocaines
- 6-alkyl-3-benzyl tropanes
- piperidine homologues of cocaine
However strict analogues of cocaine would also include such other potential combinations as phenacyltropanes & other carbon branched replacements not listed above. The term may also be loosely used to refer to drugs manufactured from cocaine or having their basis as a total synthesis of cocaine, but modified to alter their effect & QSAR. These include both intracellular sodium channel blocker anesthetics and stimulant dopamine reuptake inhibitor ligands (such as certain piperidines).
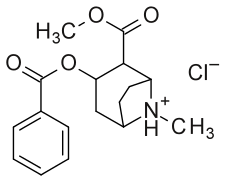
Analogs sensu stricto
Cocaine Stereoisomers
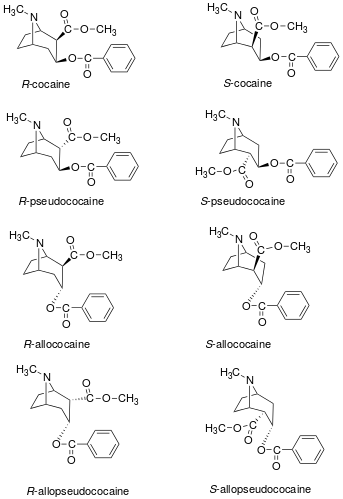
| Stereoisomer | S. Singh's alphanumeric assignation |
IC50 (nM) [3H]WIN 3542 inhibition to rat striatal membranes Mean error standard ≤5% in all cases |
IUPAC nomenclature |
|---|---|---|---|
| R-cocaine | - | 102 | methyl(1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate |
| R-pseudococaine | 172 | 15800 | |
| R-allococaine | 173 | 6160 | |
| R-allopseudococaine | 174 | 28500 | |
| S-cocaine | 175 | 15800 | |
| S-pseudococaine | 176 | 22500 | |
| S-allococaine | 177 | 9820 | methyl(1S,3S,4R,5R)-3-(benzoyl)oxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate |
| S-allopseudococaine | 178 | 67700 | |
The creation of the following analogues of cocaine have traditionally required a step which has utilized 2-CMT as an intermediate molecular product.
Benzoyl branch cleavage substitutions (excluding the exhaustive phenyl group)
C-ring 2′, 3′, 4′ (5′ & 6′) position substitutions
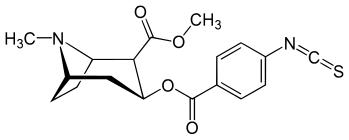
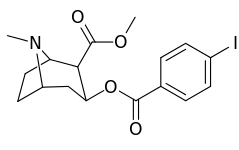
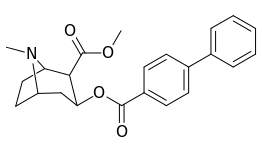
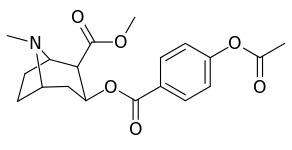
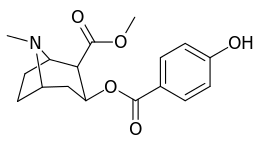
| Structure | S. Singh's alphanumeric assignation (name) |
R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
 | |||||||
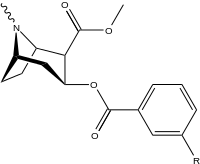
| 183a | I | 2522 ± 4 | 1052 ± 23 | 18458 ± 1073 | 0.4 | 7.3 | |
| 183b | Ph | 486 ± 63 | - | - | - | - | |
| 183c | OAc | 144 ± 2 | - | - | - | - | |
| 183d | OH | 158 ± 8 | 3104 ± 148 | 601 ± 11 | 19.6 | 3.8 | |
| (4′-Fluorococaine)[4] | F | - | - | - | - | - | |
| (Isothiocyanatobenzoylecgonine methyl ester)[5] (p-Isococ) | NCS | - | - | - | - | - |
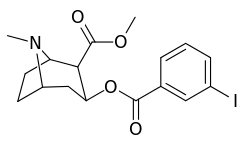
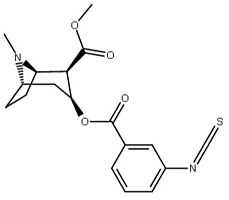
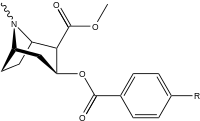
| Structure | S. Singh's alphanumeric assignation (name) |
R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
 | |||||||
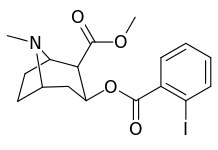
| 184a | I | 325 (IC50 value for displacement of [3H]cocaine) | - | - | - | - | |
| 184b | OH | 1183 ± 115 | 793 ± 33 | 3760 ± 589 | 0.7 | 3.2 | |
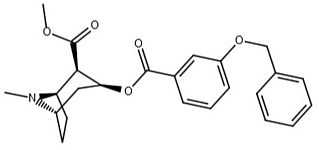
| 191 | OBn | - | - | - | - | - | |
| (m-Isococ) | NCS | - | - | - | - | - |
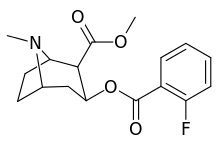
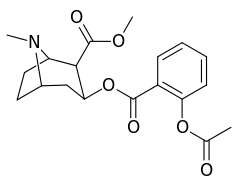
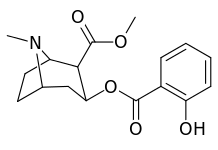
The hydroxylated 2′-OH analogue exhibited a ten fold increase in potency over cocaine.[lower-alpha 3]
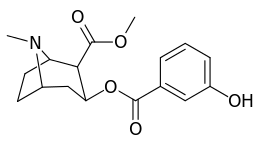
| Structure | S. Singh's alphanumeric assignation (name) |
R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
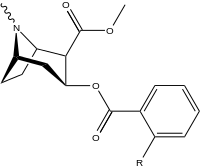 | |||||||
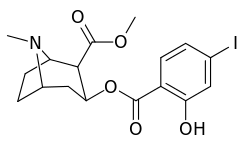
| 185a | I | 350 (IC50 value for displacement of [3H]cocaine) | - | - | - | - | |
| 185b | F | 604 ± 67 | 1770 ± 309 | 1392 ± 173 | 2.9 | 2.3 | |
| 185c (2′-Acetoxycocaine)[6] | OAc | 25 ± 4 | 143 ± 21 | 48 ± 2 | 5.7 | 1.9 | |
| 185d (2′-Hydroxycocaine)[2] | OH | 215 ± 19 | 195 ± 10 | 1021 ± 75 | 0.9 | 4.7 |

Meta-substitutions (substitutions of substitutions) or manifold ("many-fold") substituted analogues are analogues where more than one modification from the parent molecule takes place (having numerous intermediary constituents). These are created with often surprising structure–activity relationship results extrapolated therefrom. It is even a common case where two separate substitutions can each yield a weaker, lower affinity or even wholly non-efficacious compound respectively; but due to findings that often times, when used together, such two mutually inferior changes being added in tandem to one analogue has the potential to make the resultant derivative display much greater efficacy, affinity, selectivity &/or strength than even the parent compound; which otherwise was compromised by either of those two alternations when made alone.
For an exposition & allusion to this mechanism observe that the opioid oxycodone, derived from codeine, is 1.5×—1.7× the analgesic potency of morphine (an opioid to which codeine is by comparison only 8%—12% as potent relatively, or 0.17th its strength in rats); yet oxycodone's intermediates in it's synthesis from codeine are: ⅓ the potency of codeine (i.e. codeinone); 0.13 that of morphine (i.e. 14-hydroxycodeine) in rats and less in mice (to illustrate: the former even being less than the 0.17 of morphine that codeine is); with the final possible stand alone intermediate compound between codeine & oxycodone (i.e. 7,8-dihydrocodeine) being at most 150% to 200% that of codeine.[7]
| Structure | S. Singh's alphanumeric assignation (name) |
C2′=R | C3′=R | C4′=R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|---|---|
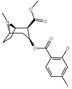 | 186 | HO | H | I | 215 ± 19 | 195 ± 10 | 1021 ± 75 | 0.9 | 4.7 |
 | (Vanillylmethylecgonine)[3] | H | OCH3 | OH | - | - | - | - | - |
| Structure | S. Singh's alphanumeric assignation (name) |
C=R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
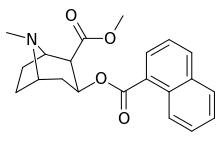
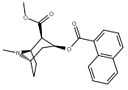 | 187 | 1-naphthalene | 742 ± 48 (IC50 value for displacement of [3H]cocaine) | - | - | - | - |
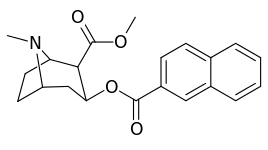
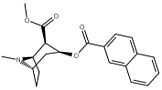 | 188 | 2-naphthalene | 327 ± 63 (IC50 value for displacement of [3H]cocaine) | - | - | - | - |
Benzoyl branch modifications
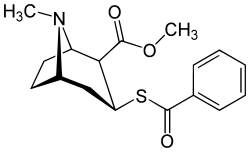
A sulfur in place of the oxygen at the benzoyl ester single bond results in a lower electronegativity than that of cocaine.
2β-substitutions
(including transesterification metabolite substitution cocaethylene)
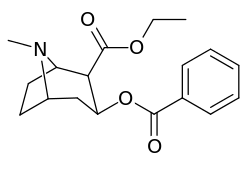
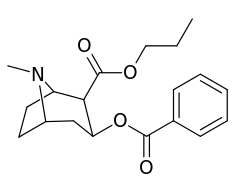
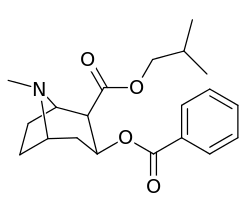
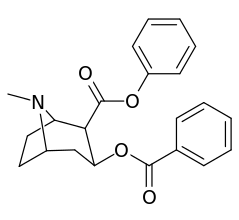
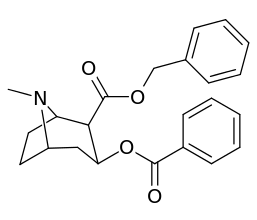
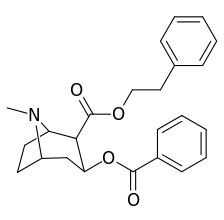
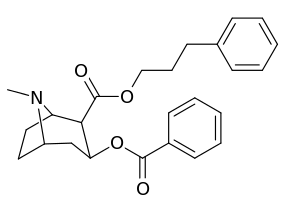
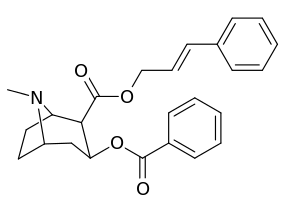
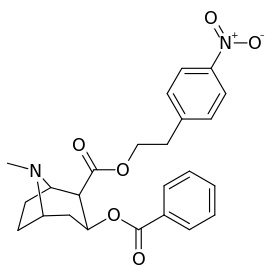
| Additional 2β-Cocaine analogue molecular structures | |
|---|---|
|
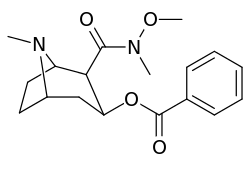
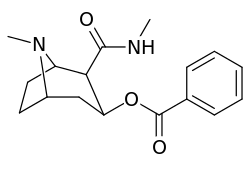
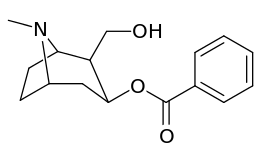
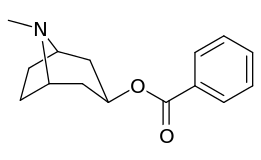
Compound 197b displayed a 1,131-fold increased selectivity in affinity for the serotonin transporter, with only slight reductions in potency for the dopamine & norepinephrine transporters.[lower-alpha 4] Whereas 197c had a 469× increase at SERT, with greater affinity for DAT than cocaine & was approximately equipotent to NET.[lower-alpha 5] 197b was 137×, and 196c 27× less potent at binding to the serotonin transporter, but both had a NET / DAT ratio that was better than cocaine.[lower-alpha 6]
| Structure | S. Singh's alphanumeric assignation (name) |
R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
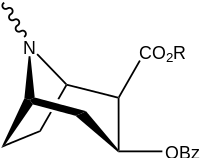 | |||||||
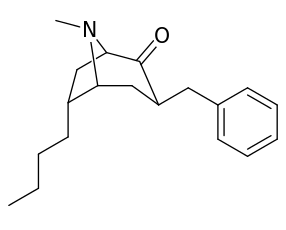
| (Cocaine) | Me | 89 ± 4.8 | 1045 ± 89 | 3298 ± 293 | 11.7 | 37.0 | |
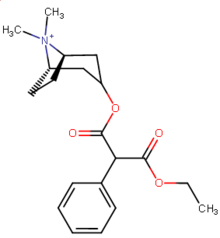
| 196a (Cocaethylene) | Et | 195 ± 45 | 5801 ± 493 | 10000 ± 751 | 29.7 | 51.3 | |
| 196b | n-Pr | 196 ± 46 | 4517 ± 430 | 6124 ± 262 | 23.3 | 31.2 | |
| 196c | i-Pr | 219 ± 48 | 25224 ± 1498 | 30384 ± 1685 | 115 | 139 | |
| 196d | Ph | 112 ± 31 | 33666 ± 3330 | 31024 ± 1909 | 300 | 277 | |
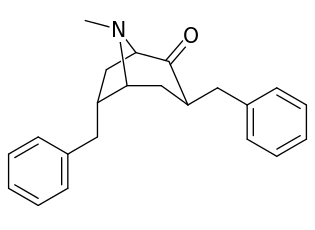
| 196e | Bn | 257 ± 14 | 302 ± 23 | 20794 ± 950 | 1.2 | 80.9 | |
| 196f | β-phenethyl | 181 ± 10 | 615 ± 52 | 19944 ± 1026 | 3.4 | 110 | |
| 196g | γ-phenylpropyl | 147 ± 19 | 374 ± 15 | 4893 ± 344 | 2.5 | 33.3 | |
| 196h | cinnamyl | 371 ± 15 | 368 ± 6.3 | 68931 ± 3476 | 1.0 | 186 | |
| 196i | p-NO2-β-phenethyl | 601 ± 28 | - | - | - | - | |
| 196j | p-Cl-β-phenethyl | 271 ± 12 | - | - | - | - | |
| 196k | p-NH2-β-phenethyl | 72 ± 7 | - | - | - | - | |
| 196l | p-NCS-β-phenethyl | 196 ± 14 | - | - | - | - | |
| 196m | p-azido-β-phenethyl | 227 ± 19 | - | - | - | - | |
| 196n | (p-NHCOCH2Br)β-phenethyl | 61 ± 6 | - | - | - | - | |
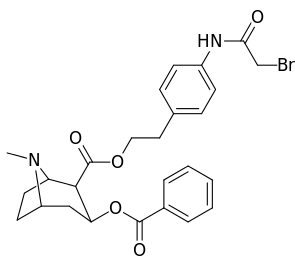
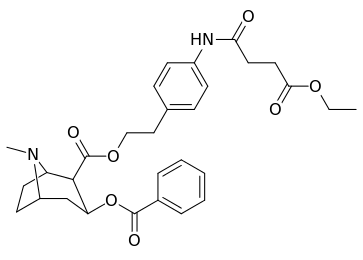
| 196o | (p-NHCO(CH2)2CO2Et)β-phenethyl | 86 ± 4 | - | - | - | - | |
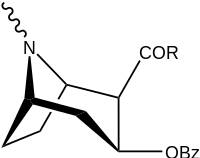 | 197a | NH2 | 753 ± 41.3 | 13725 ± 1256 | 3981 ± 229 | 18.2 | 5.3 |
| 197b | -NMe2 | 127 ± 6.36 | 143713 ± 8854 | 7329 ± 158 | 1131 | 57.7 | |
| 197c | -N(OMe)Me | 60 ± 6.4 | 28162 ± 2565 | 3935 ± 266 | 469 | 65.6 | |
| 197d | -NHMe | 2424 ± 118 | 44798 ± 2105 | 4213 ± 206 | 18.5 | 1.7 | |
| 197e (Benzoylecgonine) | -OH | 195000 | - | - | - | - | |
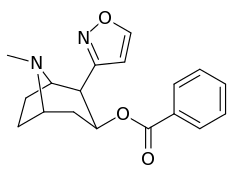
 | 197f | HOCH2- | 561 ± 149 | - | - | - | - |
| 197g (Tropacocaine) | H | 5180 ± 1160 | - | - | - | - |
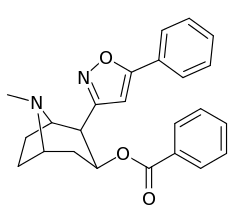
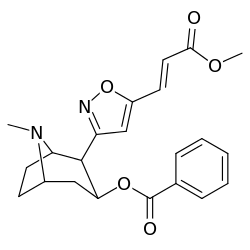
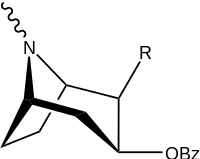
| Structure | S. Singh's alphanumeric assignation (name) |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
| (Cocaine) | (H) | 580 ± 70 | 570 ± 180 | 1.0 | |
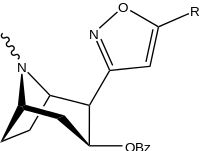 | |||||
| 198a | H | 520 ± 40 | 260 ± 70 | 0.5 | |
| 198b | CO2Et (5′-carboethoxy-) | 120 ± 10 | 290 ± 40 | 2.4 | |
| 198c | BOC | 2230 ± 220 | 1820 ± 810 | 0.8 | |
| 198d | Ph | 2000 ± 640 | 2920 ± 1620 | 1.5 | |
| 198e | CH=CHCO2Me | 3600 ± 400 | 3590 ± 1180 | 1.0 |
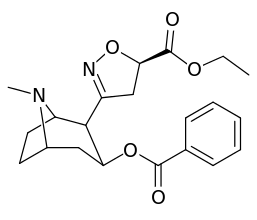
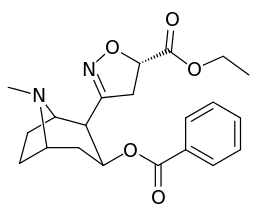
| Structure | S. Singh's alphanumeric assignation |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
| 199a | β(or R)CO2Et | 710 ± 150 | 1060 ± 340 | 1.5 | |
| 199b | α(or S)CO2Et | 5830 ± 630 | 8460 ± 620 | 1.4 |
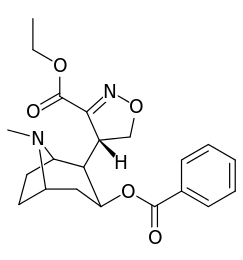
| Structure | S. Singh's alphanumeric assignation |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
| 200 | 880 ± 350 | 400 ± 140 | 0.4 |
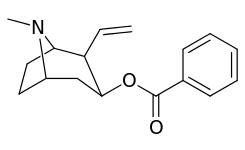
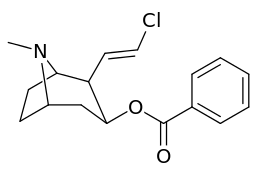
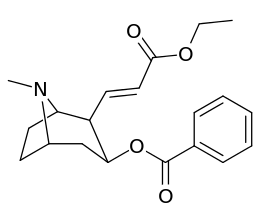
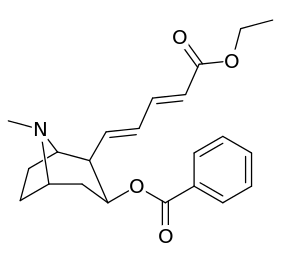
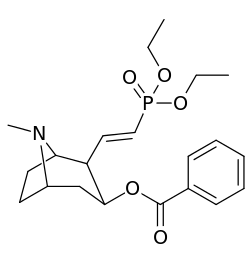
201b & 201c show significant increased potency over cocaine; whereas 201a, 201d & 201e are considerably less so. This infers the hydrogen bond acceptor at the 2β position to not necessarily be of exclusive import in creation of higher binding analogues of cocaine.
| Structure | S. Singh's alphanumeric assignation |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
| 201a | H | 1730 ± 550 | 1120 ± 390 | 0.6 | |
| 201b | Cl | 222 ± 49 | 368 ± 190 | 1.6 | |
| 201c | CO2Et | 50 ± 10 | 130 ± 10 | 2.6 | |
| 201d | CH=CHCO2Et | 1220 ± 100 | 870 ± 50 | 0.7 | |
| 201e | PO(OEt)2 | 4850 ± 470 | 5500 ± 70 | 1.1 |
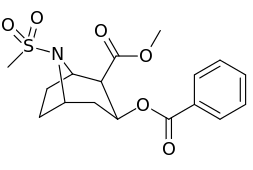
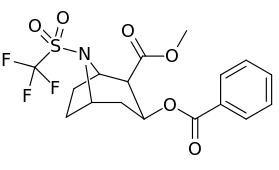
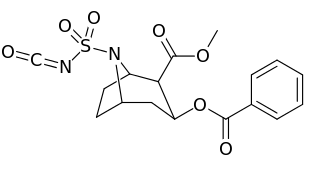
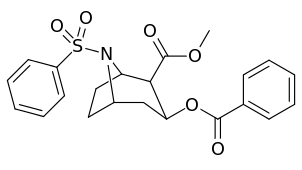
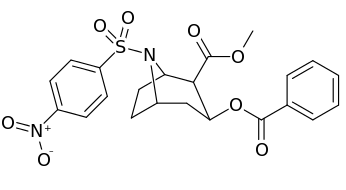
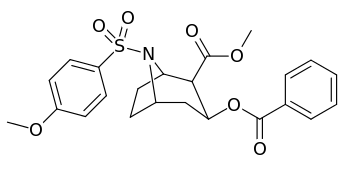
N-modifications
.svg.png)
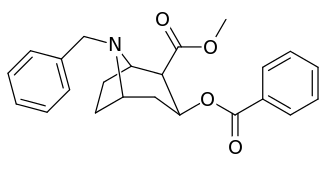
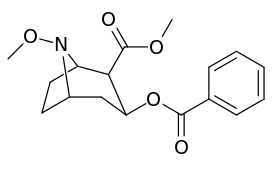
| Compound | S. Singh's alphanumeric assignation (name) |
R | [3H]WIN 35428 binding | [3H]DA
uptake |
Selectivity
uptake/binding |
|---|---|---|---|---|---|
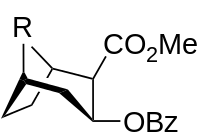 | (Cocaine) | CH3 | 102 | - | - |
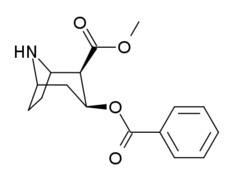
| 218 (Norcocaine) | H | 303 ± 59 | - | - | |
| 219a | Bn | 668 ± 67 | - | - | |
| 219b | Ac | 3370 ± 1080 | - | - |
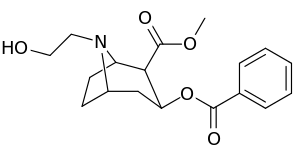
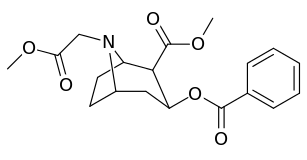
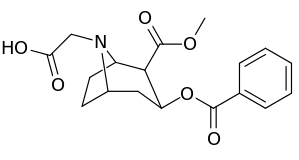
| Additional N-modified cocaine analogue molecular structures | |
|---|---|
|
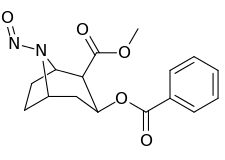
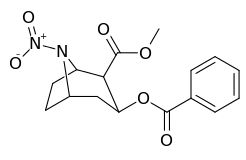
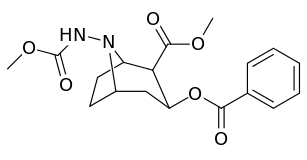
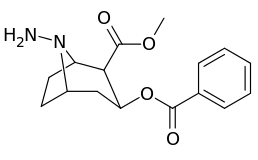
| Compound | S. Singh's alphanumeric assignation (name) |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
 | (Cocaine) | CH3 | 280 ± 60 | 320 ± 10 | 1.1 |
| 218 (Norcocaine) | H | - | - | - | |
| 219a | Bn | - | - | - | |
| 219b | Ac | - | - | - | |
| 219c | CH2CH2OH | 700 ± 100 | 1600 ± 200 | 2.3 | |
| 219d | CH2CO2CH3 | 480 ± 40 | 1600 ± 100 | 3.3 | |
| 219e | CH2CO2H | 380 ± 20 | 2100 ± 400 | 5.5 | |
| 220a | SO2CH3 (Ms) | 1290 ± 80 | 1970 ± 70 | 1.5 | |
| 220b | SO2CF3 (Tf) | 330 ± 30 | 760 ± 20 | 2.3 | |
| 220c | SO2NCO | 120 ± 10 | 160 ± 10 | 1.3 | |
| 220d | SO2Ph | 20800 ± 3500 | 61000 | 2.9 | |
| 220e | SO2C6H4-4-NO2 (nosyl) | 5720 ± 1140 | 18800 ± 90 | 3.3 | |
| 220f | SO2C6H4-4-OCH3 | 6820 ± 580 | 16400 ± 1400 | 2.4 | |
| 221a | NO | 99500 ± 12300 | 231700 ± 39500 | 2.3 | |
| 221b | NO2 | 7500 ± 900 | 21200 ± 600 | 2.8 | |
| 221c | NHCOCH3 | >1000000 | >1000000 | - | |
| 221d | NH2 | - | - | - |
Tropane fused/bridged analogues
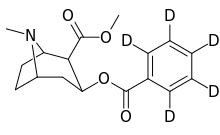
| Compound | S. Singh's alphanumeric assignation |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
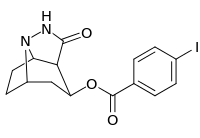 | 222 | 44900 ± 6200 | 115000 ± 15700 | 2.6 |
6/7 tropane position methoxycocaine analogues
(including pseudococaine)
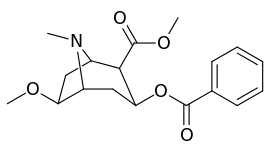
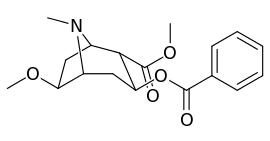
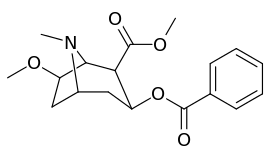
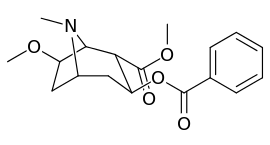
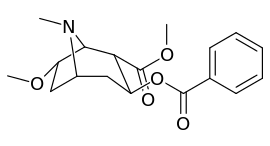
| Compound | S. Singh's alphanumeric assignation (name) |
X | Ki (nM) [3H]Mazindol binding |
Ki (nM) [3H]DA uptake |
Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
| (Cocaine) | 280 ± 60 | 320 ± 10 | 1.1 | ||
| (Pseudococaine) | 10400 ± 300 | 13800 ± 1500 | 1.3 | ||
| 225a | 2β, 6β-OCH3 | 98000 ± 12000 | 68000 ± 5000 | 0.7 | |
| 225b | 2α, 6β-OCH3 | 190000 ± 11000 | 510000 ± 110000 | 2.7 | |
| 225c | 2β, 7β-OCH3 | 4200 ± 100 | 6100 ± 200 | 1.4 | |
| 225d | 2α, 7β-OCH3 | 45000 ± 5000 | 110000 ± 4000 | 2.4 | |
| 225e | 2α, 7α-OCH3 | 54000 ± 3000 | 200000 ± 70000 | 3.7 |
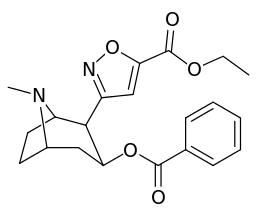
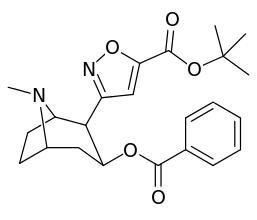
Carbon position 2′—(6′) & 2β-substitution combination analogues
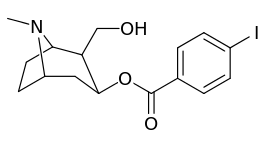
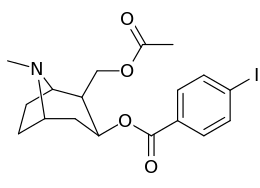
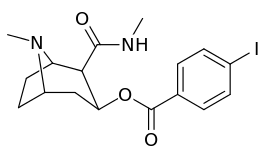
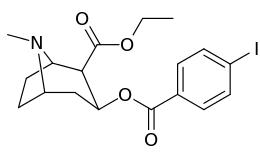
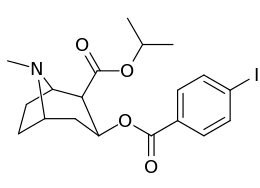
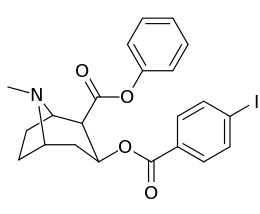
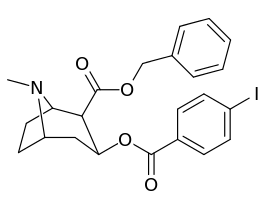
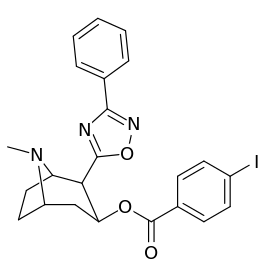
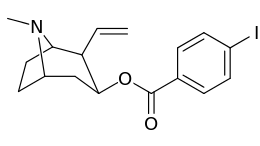
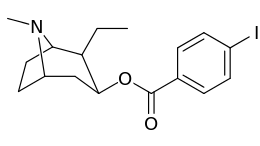
| Compound | S. Singh's alphanumeric assignation |
2β-R | C2′-R | IC50 (nM) (displacement of [3H]WIN 35428) |
|---|---|---|---|---|
| 211a | CO2OH | H | 6214 ± 1269 | |
| 211b | CH2OCOCH3 | H | 2995 ± 223 | |
| 211c | CONHCH3 | H | >100000 | |
| 211d | CO2Et | H | 2031 ± 190 | |
| 211e | CO2-i-Pr | H | 1377 ± 10 | |
| 211f | CO2Ph | H | 2019 ± 253 | |
| 211g | CO2CH2Ph | H | 4602 ± 325 | |
| 211h | 3-phenyl-1,2,4-oxadiazole | H | 3459 ± 60 | |
| 211i | CH=CH2 | H | 2165 ± 253 | |
| 211j | CH2CH3 | H | 2692 ± 486 | |
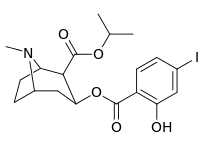 | 212 | CH2CH3 | HO | 663 ± 70 4507 ± 13 for [3H]paroxetine (5-HTT & NET) 34838 ± 796 for [3H]nisoxetine (5-HTT & NET) |
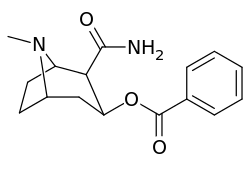
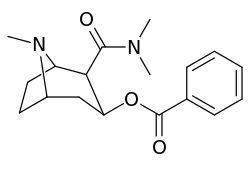
3β-Carbamoyl analogues
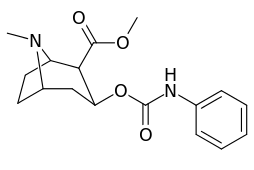
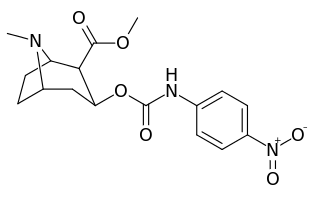
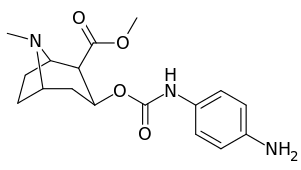
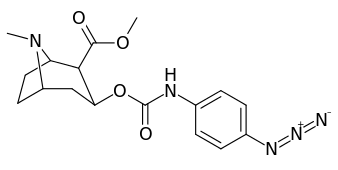
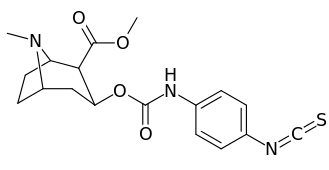
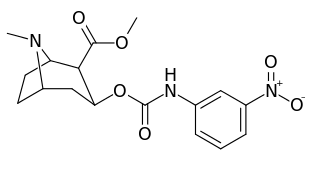
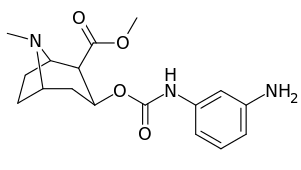
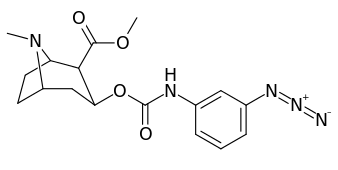
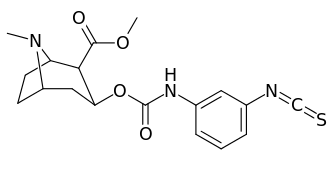
| Compound | S. Singh's alphanumeric assignation (name) |
X | IC50 (nM) inhibition of [3H]Cocaine binding (Rat Striatal Tissue) |
IC50 (nM) inhibition of [3H]DA uptake (Rat Striatal Tissue) |
Selectivity uptake/binding |
|---|---|---|---|---|---|
| (Cocaine) | (H) | 70 ± 10 | 210 ± 70 | 3.0 | |
| 223a | H | 5600 ± 700 | 52600 ± 3000 | 9.4 | |
| 223b | 4-NO2 | 1090 ± 250 | 5700 ± 1200 | 5.2 | |
| 223c | 4-NH2 | 63300 ± 12200 | >100000 | - | |
| 223d | 4-N3 | 1000 ± 240 | 1180 ± 360 | 1.2 | |
| 223e | 4-NCS | 260 ± 60 | 490 ± 80 | 1.9 | |
| 223f | 3-NO2 | 37 ± 10 | 178 ± 23 | 4.8 | |
| 223g | 3-NH2 | 2070 ± 340 | 23100 ± 900 | 11.1 | |
| 223h | 3-N3 | 630 ± 150 | 3900 ± 1590 | 6.2 | |
| 223i | 3-NCS | 960 ± 210 | 4900 ± 420 | 5.1 |
Phenyl 3-position linkage substitutions

See: List of phenyltropanes (Many phenyltropanes are derived from cocaine metabolites, such as methylecgonidine, as precursors)
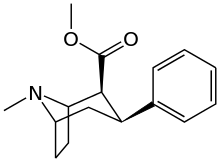
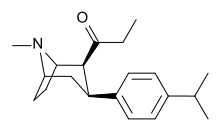
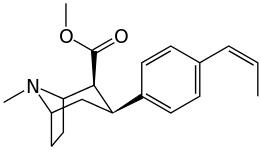
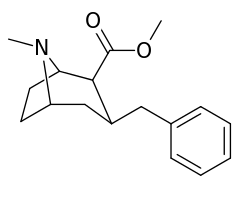
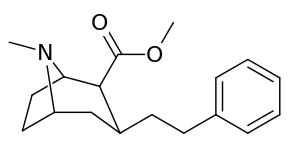
| Various phenyltropane examples | |
|---|---|
|
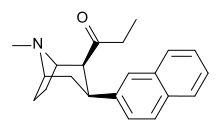
3β-Alkylphenyltropane analogues
The compound 224e, the 3β-styrene analogue, had the highest potency in its group. While 224b & 224c showed the most selectivity, with 224b having a ten-fold greater potency for the dopamine transporter than cocaine.[lower-alpha 18]
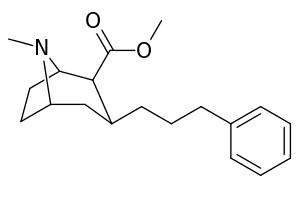
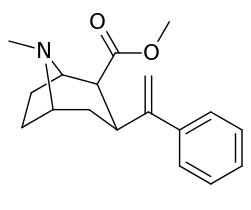
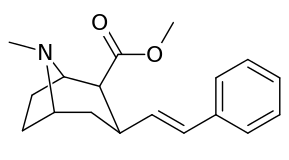
| Compound | S. Singh's alphanumeric assignation (name) |
n | IC50 (nM) [3H]Cocaine binding |
IC50 (nM) [3H]DA uptake |
Selectivity uptake/binding |
|---|---|---|---|---|---|
| (Cocaine) | 101 ± 26 | 209 ± 20 | 2.1 | ||
| 224a | 1 | 885 ± 18 | 1020 ± 52 | 1.1 | |
| 224b | 2 | 9.9 ± 0.33 | 70.5 ± 1.0 | 7.1 | |
| 224c | 3 | 344 ± 12 | 2680 ± 190 | 7.8 | |
| 224d | 71.6 ± 0.7 | 138 ± 9 | 1.9 | ||
| 224e | 2.10 ± 0.04 | 5.88 ± 0.09 | 2.8 |
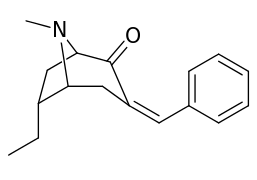
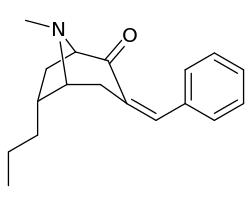
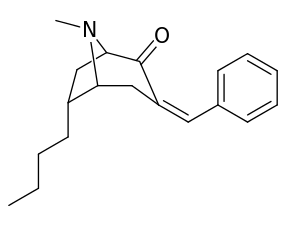
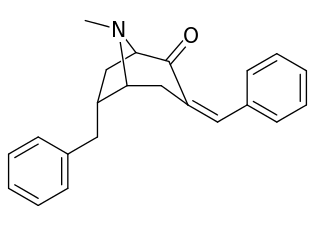
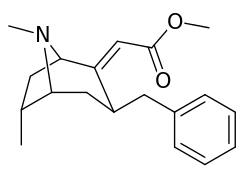
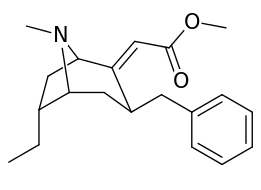
6-Alkyl-3-benzyltropane analogues
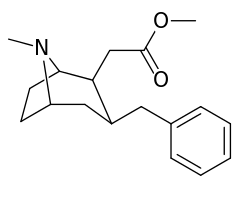
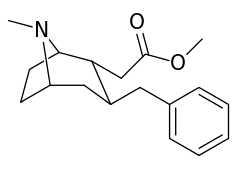
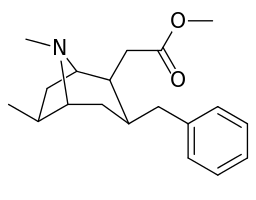
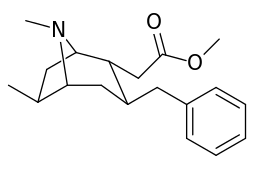
| Cocaine 229b—f series analogues | |
|---|---|
|
| Cocaine 230b—f series analogues | |
|---|---|
|
| Cocaine 231c—f series analogues | |
|---|---|
|
| Cocaine 232c, d & f series analogues | |
|---|---|
|
| Compound | S. Singh's alphanumeric assignation (name/WIN number) |
R | Ki (nM) [3H]WIN 35428 binding |
IC50 (nM) [3H]DA uptake |
Selectivity
uptake/binding |
|---|---|---|---|---|---|
| (Cocaine) | 32 ± 5 338 ± 221 | 405 ± 91 405 ± 91 | 12.6 1.2 | ||
| 11a (WIN 35065-2) | 33 ± 17 314 ± 222 | 373 ± 10 | 11.3 | ||
| (−)-229a | H | 33 ± 5 | 161 ± 100 | 4.9 | |
| 229a | H | 91 ± 10 | 94 ± 26 | 1.0 | |
| 229b | Me | 211 ± 23 | - | - | |
| 229c | Et | 307 ± 28 | - | - | |
| 229d | n-Pr | 4180 ± 418 | - | - | |
| 229e | n-Bu | 8580 ± 249 | - | - | |
| 229f | Bn | 3080 ± 277 | - | - | |
| (+)-230a | H | 60 ± 6 | 208 ± 63 | 3.5 | |
| 230a | H | 108 ± 14 | 457 ± 104 | 4.2 | |
| 230b | Me | 561 ± 64 | - | - | |
| 230c | Et | 1150 ± 135 | - | - | |
| 230d | n-Pr | 7240 ± 376 | - | - | |
| 230e | n-Bu | 19700 ± 350 | - | - | |
| 230f | Bn | 7590 ± 53 | - | - | |
| 231b | Me | 57 ± 5 | 107 ± 36 | 1.9 | |
| 231c | Et | 3110 ± 187 | - | - | |
| 231d | n-Pr | 5850 ± 702 | - | - | |
| 231f | Bn | 1560 ± 63 | - | - | |
| 232b | Me | 294 ± 29 | 532 ± 136 | 1.8 | |
| 232c | Et | 6210 ± 435 | - | - | |
| 232d | n-Pr | 57300 ± 3440 | - | - | |
| 232f | Bn | 3080 ± 277 | - | - | |
| 241 | Bn | 4830 ± 434 | - | - |
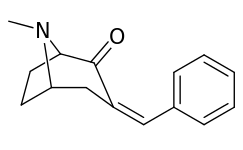
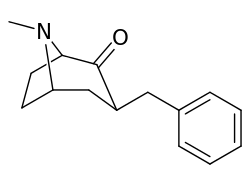
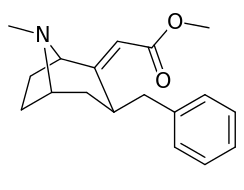
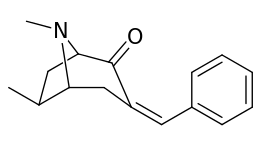
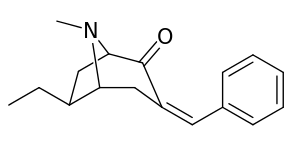
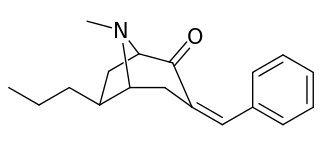
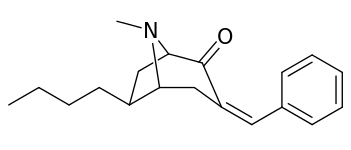
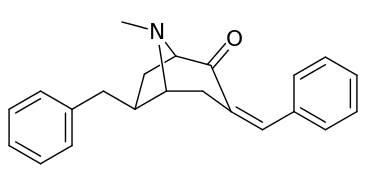
| 6α-isomers (benzylidene 6-alkyl-3-benzyl cocaine analogues) | |
|---|---|
|
| 6β-isomers (exo) ↑vide supra↑ | |
|---|---|
|
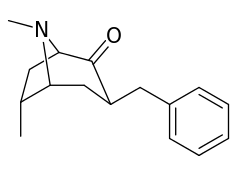
| 3β-benzyl derivatives ⇡vide supra⇡ | |
|---|---|
|
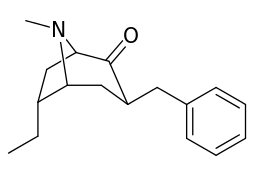
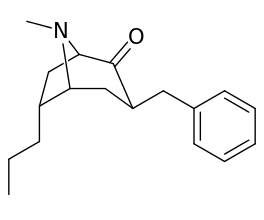
| intermediate alkylidene esters ⇡vide supra⇡ | |
|---|---|
|
| Compound | S. Singh's alphanumeric assignation |
R | Ki (nM) [3H]WIN 35428 binding |
IC50 (nM) [3H]DA uptake |
Selectivity
uptake/binding |
|---|---|---|---|---|---|
| 6α-isomers | |||||
| 237b | Me | - | - | - | |
| 237c | Et | - | - | - | |
| 237d | n-Pr | - | - | - | |
| 237e | n-Bu | - | - | - | |
| 237f | Bn | - | - | - | |
| 6β-isomers (exo) | |||||
| 238b | Me | - | - | - | |
| 238c | Et | - | - | - | |
| 238d | n-Pr | - | - | - | |
| 238e | n-Bu | - | - | - | |
| 238f | Bn | - | - | - | |
| 3β-benzyl derivatives | |||||
| 239a | H | - | - | - | |
| 239b | Me | - | - | - | |
| 239c | Et | - | - | - | |
| 239d | n-Pr | - | - | - | |
| 239e | n-Bu | - | - | - | |
| 239f | Bn | - | - | - | |
| intermediate alkylidene esters | |||||
| 240a | H | - | - | - | |
| 240b | Me | - | - | - | |
| 240c | Et | - | - | - | |
| 240d | n-Pr | - | - | - | |
| 240e | n-Bu | - | - | - | |
| 240f | Bn | - | - | - | |
Piperidine cocaine-homologues
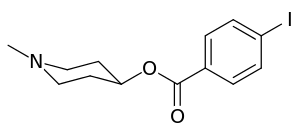
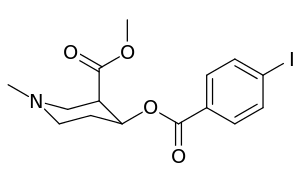
| Compound | S. Singh's alphanumeric assignation (name) |
R | IC50 (nM) |
|---|---|---|---|
| (Cocaine) | 249 ± 37 | ||
| 183a | 2522 ± 4 | ||
| 242 | H | 11589 ± 4 | |
| 243 | CO2CH3 | 8064 ± 4 |
Cocaine hapten analogues
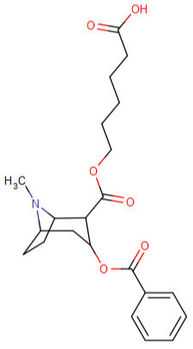
| Compound | S. Singh's alphanumeric assignation (name) |
|---|---|
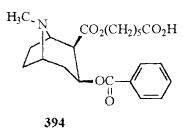 | 394 |
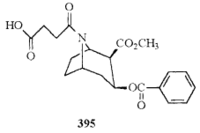 | 395 |
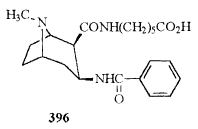 | 396 |
| Compound | S. Singh's alphanumeric assignation (name) |
R |
|---|---|---|
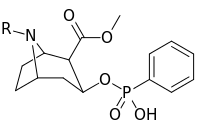 | ||
| 401a | CH3 | |
| 401b | (CH2)5CO2H | |
| 401c | CH2CO2H | |
| 401d | COCH2CH2CO2H | |
| 401e | H | |
| 401f | CH2CH2Br | |
| 385g | (CH2)2NHCO(CH2)2CONH2 | |
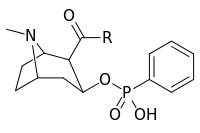 | ||
| 402a | O(CH2)4NHCO(CH2)2CO2…2,3-dihydro-1H-isoindole-1,3-dione | |
| 402b | OH | |
| 402c | O(CH2)2…1,4-xylene…NH2 | |
| 402d | NH(CH2)5CO2H | |
| 402e | O(CH2)4NHCO(CH2)2CONH2 | |
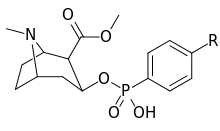 | ||
| 403a | NH2 | |
| 403b | NHCOCH2Br | |
| 403c | NHCO(CH2)3CO2H | |
| 403d | (CH2)3NHCO(CH2)2CONH2 |
| Compound | Name |
|---|---|
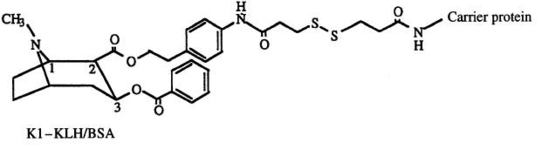 | |
| K1-KLH/BSA[11] | |
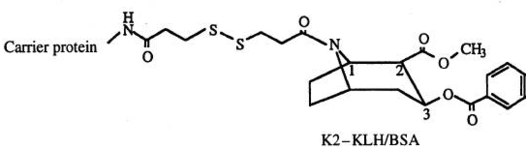 | |
| K2-KLH/BSA |
Structural/Functional intermediate analogues
Tropane (non-ecgonine) analogues
pFBT: tropane.png) Zatosetron:
Zatosetron:  Tropanserin:
Tropanserin:  Bemesetronum:
Bemesetronum: 
- 3-(p-Fluorobenzoyloxy)tropane (30% stimulant potency of cocaine & equipotent as an anaesthetic)
- Zatosetron (anxiolytic & antinauseant 5HT3 receptor antagonist)
- Tropanserin (migraine medication, potent & selective 5HT3 antagonist)
- Bemesetronum (antiemetic, mechanisms related to oxytocin function, serotonin D-receptors, cholinoreceptors of the muscarinic or nicotinic kind and histamine H1-receptors[13])
Convolamine:  Phyllalbine:
Phyllalbine: 
Similarly, many natural tropane alkaloids found in plants of various families have benzoyl tropane structures. Including; catuabine, convolamine of the convolvulaceae & phyllalbine of euphorbiaceae (Phyllanthus discoïdes) families. The latter is a a central and peripheral sympathomimetic drug.[14]
Other tropanyl compounds (naturally found or otherwise) begin to fall outside the spectrum of functional analogues to cocaine altogether; having negligible affinity of any kind for the monoamine system. Compare for example ipratropium, mirisetron, technepine, levomepate or scopolamine & atropine. Many of the natural varieties being deliriants.
Piperidine Analogues
See: List of methylphenidate analogues
Many of the piperidine analogues of cocaine serve as the 'missing link' between the cocaine structure and that of the methylphenidate class of drugs. For example DMNPC preserves an orientation similar to the phenyltropanes, but is a structural isomer of methylnaphthidate.
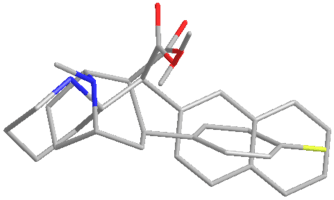
The above depicts the 3D structure of the above mentioned methylnaphthidate shown with the same modeling for the cocaine derivative WIN 35428, a simple phenyltropane with a short addition to its C4 position. This overlay shows the closeness of where the two hold their respective oxygen and nitrogens in their structure (also their benzene & cycloalkane ring formations) and is meant to convey a sense of their similarity for binding to MAT. Correspondingly most other monoamine reuptake inhibitors bind to the dopamine transporter substrate recognition site at Tm loci 1, 7 & 10—12; whereas cocaine & methylphenidate similarly share the 1 & 7 places, but diverge from the usual ligand site of the latter and instead cohabit the 9—11 loci.[lower-alpha 25] Site-directed mutagenesis techniques have elucidated that the hydrophobic putative transmembrane regions at one & seven contain aspartate and serine residues, and that the carboxyl-group interacts with the former aspartic acid residue 79 which engages with cocaine & methylphenidate's protonated nitrogen at the transporter.[lower-alpha 26]
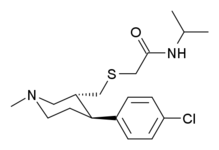
-CPCA.svg.png)
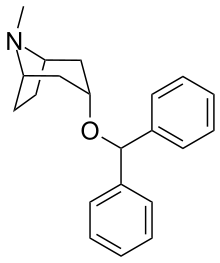
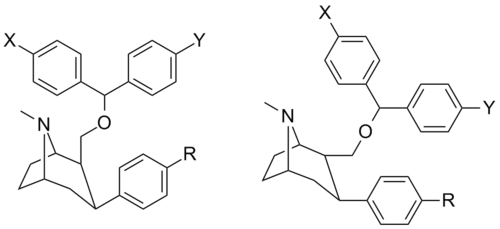
Benztropine (3α-Diphenylmethoxy Tropane) Analogs
- Benzatropine
- Difluoropine (more selective as a DARI than cocaine. Also an anticholinergic & antihistamine)
- AHN 1-055 Same structure as for benztropine but 4′,4′-bisfluorinated.
- GA 103 N-phenylpropyl bis-4-fluorobenztropine
- JHW 007[16] N-(n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane
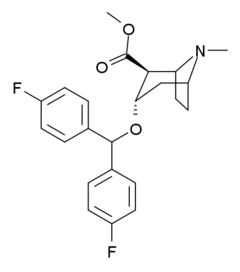
"Difluoropine" is not a phenyltropane but actually belongs to the benzatropine family of DRIs.
In certain respects these are important because they share SAR overlap with GBR 12909 and related analogs.
SARs have shown that 4′,4′-difluorination is an excellent way to boost DAT activity of benztropine, and gives excellent selectivity over the SERT and the NET.[17][18]
Furthermore, replacing the N-Me with, e.g. n-phenylpropyl helps to bring muscarinic activity down to something that is the same as DRI affinity.[17]
This is remarkable considering unmodified (native) benztropine is 60 times more active as an anticholinergic than as a dopaminergic.[17]
M1 receptor considerations aside, analogues of this benztropine class still won't substitute for cocaine, and have no propensity to elevate locomotor activity.
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | Ki (nM) [3H]WIN 35428 binding |
IC50 (nM) [3H]DA uptake |
Selectivity
uptake/binding |
|---|---|---|---|---|---|---|
| (Cocaine) | 388 ± 47 | - | - | |||
| (GBR 12909) | 11.6 ± 31 | - | - | |||
| (Benztropine) | H | H | 118 ± 9 | 403 ± 115 | 3.4 | |
| 249a | 4′-F | H | 32.2 ± 10 | 48 | 1.5 | |
| 249b | 4′-F | 4′-F | 11.8 ± 1 | 71 | 6.0 | |
| 249c | 3′,4′-di-F | H | 27.9 ± 11 | 181 ± 45.7 | 6.5 | |
| 249d | 4′-Cl | H | 30.0 ± 12 | 115 | 3.8 | |
| 249e | 4′-Cl | 4′-Cl | 20.0 ± 14 | 75 | 3.8 | |
| 249f | 3′,4′-di-Cl | H | 21.1 ± 19 | 47 | 2.2 | |
| 249g | 3′,4′-di-Cl | F | 18.9 ± 14 | 24 | 1.3 | |
| 249h | 4′-Br | H | 37.9 ± 7 | 29 | 0.8 | |
| 249i | 4′-Br | 4′-Br | 91.6 | 34 | 0.4 | |
| 249j | 4′-NO2 | H | 197 ± 8 | 219 | 1.1 | |
| 249k | 4′-CN | H | 196 ± 9 | 222 | 1.1 | |
| 249l | 4′-CF3 | H | 635 ± 10 | 2155 | 3.4 | |
| 249m | 4′-OH | H | 297 ± 13 | 677 | 2.3 | |
| 249n | 4′-OMe | H | 78.4 ± 8 | 468 | 6.0 | |
| 249o | 4′-OMe | 4′-OMe | 2000 ± 7 | 2876 | 1.4 | |
| 249p | 4′-Me | H | 187 ± 5 | 512 | 2.7 | |
| 249q | 4′-Me | 4′-Me | 420 ± 7 | 2536 | 6.0 | |
| 249r | 4′-Et | H | 520 ± 8 | 984 | 1.9 | |
| 249s | 4′-t-Bu | H | 1918 | 4456 | 2.3 | |
| 250a | 3′-F | H | 68.5 ± 12 | 250 ± 64.7 | 3.6 | |
| 250b | 3′-F | 3′-F | 47.4 ± 1 | 407 ± 63.9 | 8.6 | |
| 250c | 3′-Cl | H | 21.6 ± 7 | 228 ± 77.1 | 10.5 | |
| 250d | 3′-CF3 | H | 187 ± 5 | 457 ± 72.0 | 2.4 | |
| 251a | 2′-F | H | 50.0 ± 12 | 140 ± 17.2 | 2.8 | |
| 251b | 2′-Cl | H | 228 ± 9 | 997 ± 109 | 4.4 | |
| 251c | 2′-Me | H | 309 ± 6 | 1200 ± 1.64 | 3.9 | |
| 251d | 2′-NH2 | H | 840 ± 8 | 373 ± 117 | 0.4 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | IC50 (nM) DAT (Binding of [3H]WIN 35428) |
IC50 (nM) 5-HTT (Binding of [3H]Citalopram) |
Selectivity 5-HTT/DAT |
|---|---|---|---|---|---|---|
| (benztropine) | 312 ± 1.1 | 24100 ± 14800 | 77.2 | |||
| (WIN 35428) | 12.9 ± 1.1 | 160 ± 20 | 12.4 | |||
| R-256 | 2040 ± 283 | 1460 ± 255 | 0.7 | |||
| S-257a | H | H | 33.5 ± 4.5 | 10100 ± 1740 | 301 | |
| S-257b | H | F | 13.2 ± 1.9 | 4930 ± 1200 | 373 | |
| S-257c (difluoropine) | F | F | 10.9 ± 1.2 | 3530 ± 1480 | 324 | |
| S-257d | H | Cl | 15.8 ± 0.95 | 5960 ± 467 | 377 | |
| S-257e | Cl | Cl | 91.4 ± 0.85 | 3360 ± 1480 | 36.8 | |
| S-257f | H | Br | 24.0 ± 4.6 | 5770 ± 493 | 240 | |
| S-257g | Br | Br | 72.0 ± 3.65 | 2430 ± 339 | 33.7 | |
| S-257h | H | I | 55.9 ± 10.3 | 9280 ± 1640 | 166 | |
| S-257i | Br | I | 389 ± 29.4 | 4930 ± 82 | 12.7 | |
| S-257j | I | I | 909 ± 79 | 8550 ± 442 | 9.4 | |
| S-257k | H | Me | 49.5 ± 6.0 | 13200 | 266 | |
| S-257l | Me | Me | 240 ± 18.4 | 9800 ± 2680 | 40.8 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | n | IC50 (nM) DAT (Binding of [3H]WIN 35428) |
IC50 (nM) 5-HTT (Binding of [3H]Citalopram) |
Selectivity 5-HTT/DAT |
|---|---|---|---|---|---|---|
| 258a | 20.3 ± 3.5 | - | - | |||
| 258b | H | 1 | 223 ± 53 | 4970 ± 700 | 22.3 | |
| 258c | H | 3 | 22.0 ± 11.9 | 19.7 ± 3 | 0.9 | |
| 258d | Br | 3 | 80.2 ± 8.8 | 234 ± 0.5 | 2.9 | |
| 258e | I | 3 | 119 ± 11 | 2200 ± 1250 | 18.5 | |
| 258f | H | 5 | 99.0 ± 28 | 550 ± 63 | 5.5 | |
| 259 | 616 ± 88 | 55200 ± 20000 | 89.3 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | Ki (nM) DAT (Binding of [3H]WIN 35428) |
IC50 (nM) 5-HTT (Uptake of [3H]DA) |
Selectivity uptake/binding |
|---|---|---|---|---|---|
| 260 | H | 11.2 ± 11 | 9.7 | 0.9 | |
| 261a | 3-phenylpropyl | 41.9 ± 11 | 230 | 5.5 | |
| 261b | indole-3-ethyl | 44.6 ± 11 | 1200 | 26.9 | |
| 261c | 4-phenylbutyl | 8.51 ± 14 | 39 | 4.6 | |
| 261d | 4-(4′-nitrophenyl)butyl | 20.2 ± 11 | 650 | 32.2 | |
| 261e | 3-(4′-fluorophenyl)propyl | 60.7 ± 12 | - | - | |
| 262a | n-butyl | 24.6 ± 8 | 370 | 15.0 | |
| 262b | cyclopropylmethyl | 32.4 ± 9 | 180 | 5.5 | |
| 262c | allyl | 29.9 ± 10 | 14 | 0.5 | |
| 262d | benzyl | 82.2 ± 15 | 290 | 3.5 | |
| 262e | 4-fluorobenzyl | 95.6 ± 10 | 200 | 2.1 | |
| 262f | cinnanyl | 86.4 ± 12 | 180 | 2.1 | |
| 262g | [bis(4-fluorophenyl)methoxy]ethyl | 634 ± 23 | - | - | |
| 262h | [(4-nitrophenyl)phenylmethoxy]ethyl | 57.0 ± 17 | - | - | |
| 263 | acetyl | 2340 | 4600 | 2.0 | |
| 264 | formyl | 2020 ± 13 | 5400 | 2.7 | |
| 265a | Ts | 0% (inhibition at 10 µM) | - | - | |
| 265b | Ms | 18% (inhibition at 10 µM) | - | - | |
| 266 | 108 ± 12 | 130 | 1.2 | ||
| Compound | S. Singh's alphanumeric assignation (name) |
IC50 (nM) DAT (Binding of [3H]WIN 35428) |
IC50 (nM) 5-HTT (Binding of [3H]Citalopram) | |
|---|---|---|---|---|
| R/S-268 | 2β,3β | >10000 | >1660 | |
| R/S-269 | 2α,3β | 20300 | >1660 | |
| R/S-270 | 2α,3α | 22300 | >1660 | |
| R/S-271 | 2β,3α | 520 | >1660 |
Bicyclic Amine Analogues
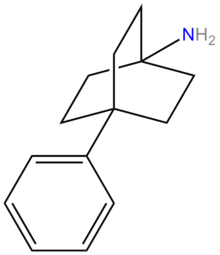
Quinuclidine Analogues
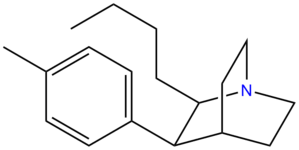
Miscellaneous loosely analogous stimulants
Benzoates
(Structures with both stimulant & local anesthetic effects)
- Dimethocaine/Larocaine (DMC) (An aniline)
- Nitracaine
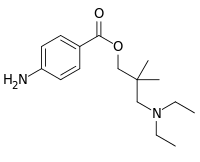
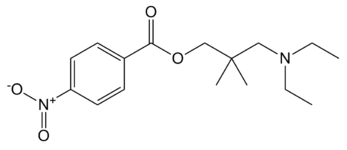
See some of Robert Clarke's contributions
Chromen-2-one
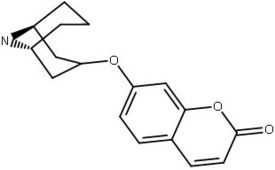
| Compound | DA-uptake IC50(μM) | NA-uptake IC50(μM) | 5-HT-uptake IC50(μM) |
|---|---|---|---|
| 7-(((1R,3r,5S)-9-Azabicyclo[3.3.1]nonan-3-yl)oxy)-2H-chromen-2-one | 0.0013 | 0.24 | 0.076 |
| 7-(((1R,3r,5S)-9-Methylazabicyclo[3.3.1]nonan-3-yl)oxy)-2H-chromen-2-one | 0.0029 | 0.15 | 0.27 |
Organochlorides
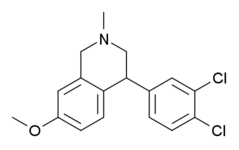
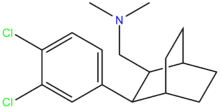
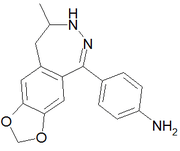
2,3-Benzodiazepines
Phenethylamines
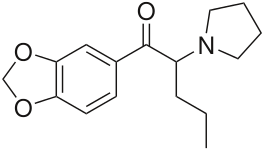
Many phenethylamines are dopamine releasers, however, certain drugs of the family inhibit dopamine reuptake & transport which may be loosely classed as cocaine analogs. Dependent upon their specific configurations.
Adamantanes

Naphthyridines
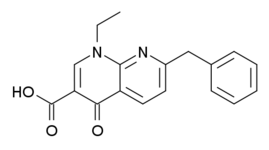
Being a carboxylic acid, amfonelic acid could potentially be used as a carboxylate for the protonation to the free base of another compound; even conceivably creating a 'cocaine amfonelate' or 'cocaine AFA' as opposed to cocaine HCl, cocaine citrate or cocaine HBr et cetera wherein such a case it's conjugate used to form it as a salt would additionally be dopaminergic.
Quinazolinamines
(Allosteric functional DAT reuptake inhibitors)

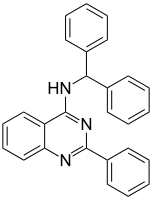 cf. the benztropine phenyltropanes:
cf. the benztropine phenyltropanes:
SoRI-20041 is a functional, but not structural, cocaine analog which violates traditional structure analog categorization in its case that it has an entirely other binding site. It is however an analog to cocaine in the sense that it functions as a partial DARI on DAT, although doing so when said DAT is compromised by amphetamine-type mediated release of DA. Something unaugmented cocaine cannot do. It nevertheless performs the role of an analogous adjunct to cocaine's function for phosphorylated DAT. It is however worth noting that as for its structure, it displays a certain degree of shared conformation with the benztropine phenyltropanes.
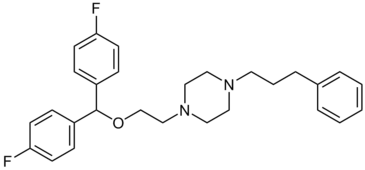
Piperazines
(Aryl-1,4-dialkyl piperazines)
GBR compounds were derived from the benztropines by replacing their tropane nucleus with a piperazine ring.[lower-alpha 32]
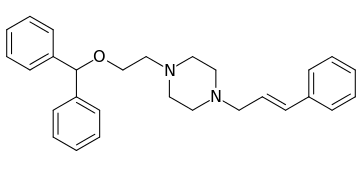
| Compound | S. Singh's alphanumeric assignation (name) |
R | Ki (nM) [3H]WIN 35425 |
IC50 (nM) [3H]DA |
Selectivity uptake/binding |
|---|---|---|---|---|---|
| (Cocaine) | 224 ± 3.4 | 208 ± 7.4 | 0.93 | ||
| (WIN 35428) | 24 ± 3.1 | 14 ± 1.8 | 0.58 | ||
| (DA) | 10000 ± 2400 | 44 ± 5.3 | 0.004 | ||
| 279 (GBR 12909/Vanoxerine) | 27 ± 4.1 | 0.21 ± 0.06 | 0.007 | ||
| 282 (GBR 12783) | H | 12 ± 1.2 (IC50 for inhibiting [3H]methylphenidate) | - | - | |
| 284a | 3-NH2 | 12 ± 1.0 | 7 ± 3.5 | 0.58 | |
| 284b | 3-NCS | 160 ± 17 | 106 ± 37 | 0.67 | |
| 284c | 4-NH2 | 11 ± 0.7 | 1.6 ± 0.2 | 0.14 | |
| 284d | 4-NO2 | 26 ± 9.3 | 2.7 ± 0.1 | 0.10 | |
| 284e | 4-NCS | 159 ± 12 | 26 ± 1.8 | 0.16 | |
| 284f | 4-maleimide | 2327 ± 1000 | 476 ± 61 | 0.20 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | X-Y (289-290) R1 (298) |
IC50 (nM) [3H]GBR 12935 binding |
IC50 (nM) [3H]DA uptake |
IC50 (nM) [3H]5-HT uptake |
Selectivity [3H]DA uptake/DAT binding |
Selectivity [3H]5-HT/[3H]DA uptake |
|---|---|---|---|---|---|---|---|---|
| (Cocaine) | 660 ± 30 (Ki value) | 478 ± 25 | 304 ± 10 | 0.72 | 0.64 | |||
| (GBR 12935) | 4.1 ± 0.6 | 3.7 ± 0.4 | 289 ± 29 | 0.90 | 78.1 | |||
| 279 (GBR 12909) | 5.5 ± 0.4 | 4.3 ± 0.3 | 73 ± 1.5 | 0.78 | 17.0 | |||
| 289a | H | C-C | 21 ± 1.0 | 9.6 ± 1.5 | 1720 ± 70 | 0.46 | 179 | |
| 289b | F | C-C | 40 ± 1 | 15 ± 2 | 459 ± 26 | 0.37 | 30.6 | |
| (-)289b (2S,5R) | F | C-C | 3.6 ± 0.14 | 8.1 ± 0.3 | - | 2.25 | - | |
| (+)289b (2R,5S) | F | C-C | 125 ± 7.0 | 87 ± 4.1 | - | 0.70 | - | |
| 289c | H | C=C | 103 ± 13 | 20 ± 4 | 2680 ± 122 | 0.19 | 134 | |
| 289d | F | C=C | 23 ± 3 | 28 ± 5 | 1180 ± 404 | 1.22 | 42.1 | |
| 290a (LR1111) | H | C-C | 7.9 ± 1.7 | 7.2 ± 0.5 | 34100 ± 359 | 0.91 | 4736 | |
| 290b | F | C-C | 4.4 ± 0.4 | 3.4 ± 0.4 | 112 ± 24 | 0.77 | 32.9 | |
| 290c | H | C=C | 8.6 ± 1.1 | 0.6 ± 0.1 | 503 ± 103 | 0.07 | 838 | |
| 290d | F | C=C | 2.6 ± 0.4 | 3.4 ± 0.4 | 234 ± 10 | 1.31 | 68.8 | |
| 291 | 286 ± 8 | 87 ± 5 | 3150 ± 491 | 0.30 | 36.2 | |||
| 292 | 864 ± 91 | 93 ± 6 | 1590 ± 60 | 0.11 | 17.1 | |||
| 293 | 27 ± 4 | 18 ± 1 | 2450 ± 57 | 0.67 | 136 | |||
| 294 | 169 ± 5 | 83 ± 7 | 1890 ± 268 | 0.49 | 22.8 | |||
| 295 | 80 ± 6 | 35 ± 2 | 376 ± 19 | 0.44 | 10.7 | |||
| 296 | 74 ± 5 | 57 ± 10 | 2860 ± 45 | 0.77 | 50.2 | |||
| 297 | 20 ± 0.7 | 9.3 ± 1.8 | 1480 ± 69 | 0.46 | 159 | |||
| (-)298a | H | H | 5.1 ± 0.4 | 0.7 ± 0.05 | 986 ± 34 | 0.14 | 1409 | |
| (+)298a | H | H | 747 ± 163 | 127 ± 10 | 3210 ± 450 | 0.17 | 25.3 | |
| (-)298b | F | H | 104 ± 8 | 29 ± 2 | 20100 ± 2400 | 0.28 | 693 | |
| (-)298c | H | OH | 222 ± 13 | 31 ± 0.1 | 857 ± 17 | 0.14 | 27.6 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | R1 | IC50 (nM) [125I]RTI-55 binding DAT |
IC50 (nM) [125I]RTI-55 binding 5-HTT |
IC50 (nM) reuptake [3H]DA |
IC50 (nM) reuptake [3H]5-HT |
Selectivity binding 5-HTT/DAT |
Selectivity uptake [3H]5-HT/[3H]DA |
|---|---|---|---|---|---|---|---|---|---|
| (GBR 12935) | C6H5 | 3.7 ± 0.3 | 623 ± 13 | 3.7 ± 0.4 | 298 ± 29 | 168 | 80.5 | ||
| 304a | 2-thienyl | 5.2 ± 0.3 | 842 ± 30 | 9.7 ± 0.2 | 1990 ± 58 | 162 | 205 | ||
| 304b | 2-furyl | 6.5 ± 0.2 | 1520 ± 47 | 8.5 ± 0.5 | 2550 ± 87 | 34 | 300 | ||
| 304c | 2-pyridyl | 78 ± 4 | 2420 ± 65 | 70 ± 6 | 3700 ± 148 | 31.0 | 52.8 | ||
| 279 (GBR 12909) | C6H5 | 3.7 ± 0.4 | 126 ± 5 | 7.3 ± 0.2 | 73 ± 2 | 34.0 | 10.0 | ||
| 305a | 2-thienyl | 3.3 ± 0.1 | 105 ± 2 | 6.1 ± 0.7 | 335 ± 17 | 31.8 | 54.9 | ||
| 305b | 2-furyl | 5.9 ± 0.3 | 204 ± 7 | 7.9 ± 0.5 | 412 ± 9 | 34.6 | 52.1 | ||
| 305c | 2-pyridyl | 16 ± 0.2 | 2800 ± 139 | 20 ± 0.8 | 6520 ± 293 | 175 | 326 | ||
| 282 (GBR 12783) | C6H5 | - | - | - | - | - | - | ||
| 306a | 2-thienyl | 6.4 ± 0.3 | 1170 ± 31 | 10 ± 0.7 | 2020 ± 141 | 183 | 202 | ||
| 306b | 2-furyl | 5.0 ± 0.3 | 1840 ± 59 | 9.6 ± 0.3 | 2700 ± 136 | 368 | 281 | ||
| 306c | 2-pyridyl | 44 ± 3 | 2670 ± 66 | 64 ± 2 | 3620 ± 179 | 60.7 | 56.6 | ||
| 283 (GBR 13069) | C6H5 | 0.9 ± 0.1 | 135 ± 7 | 11 ± 0.6 | 576 ± 32 | 150 | 52.4 | ||
| 307a | 2-thienyl | 2.2 ± 0.1 | 88 ± 2 | 13 ± 1.4 | 374 ± 17 | 40.0 | 28.8 | ||
| 307b | 2-furyl | 1.8 ± 0.3 | 109 ± 4 | 7.2 ± 0.4 | 442 ± 23 | 60.5 | 61.4 | ||
| 307c | 2-pyridyl | 13.6 ± 0.2 | 334 ± 12 | 14.5 ± 1.9 | 666 ± 21 | 24.5 | 45.9 | ||
| 308a | H | CH2…2-methyl-1-benzothiophene | 18.1 ± 1 | 2420 ± 109 | 19 ± 1 | 3520 ± 289 | 134 | 185 | |
| 308b | F | CH2…2-methyl-1-benzothiophene | 4.1 ± 1.1 | 495 ± 18 | 34 ± 2 | 1230 ± 40 | 121 | 36.2 | |
| 309a | H | CH2…2-methyl-1-benzofuran | 17 ± 0.5 | 1890 ± 48 | 22 ± 0.7 | 3040 ± 213 | 111 | 138 | |
| 309b | F | CH2…2-methyl-1-benzofuran | 6.4 ± 0.2 | 286 ± 10 | 18.6 ± 0.6 | 767 ± 27 | 44.7 | 41.2 | |
| 310a | H | CH2…2-methyl-1H-indole | 1.1 ± 0.1 | 668 ± 39 | 8.8 ± 0.7 | 2120 ± 166 | 607 | 241 | |
| 310b | F | CH2…2-methyl-1H-indole | 0.7 ± 0.1 | 119 ± 5 | 13 ± 0.2 | 506 ± 23 | 170 | 38.9 | |
| 311a | H | CH2…2-methyl-1H-1,3-benzodiazole | 46 ± 1 | 1884 ± 72 | 37 ± 2 | 4076 ± 221 | 41.0 | 110 | |
| 311b | F | CH2…2-methyl-1H-1,3-benzodiazole | 15 ± 0.2 | 256 ± 7 | 20 ± 0.8 | 797 ± 43 | 17.1 | 39.8 | |
| 312a | H | H2C…2-methylquinoline | 199 ± 5 | 1990 ± 5 | 192 ± 8 | 4120 ± 212 | 10.0 | 21.5 | |
| 312b | F | H2C…2-methylquinoline | 56 ± 1 | 51 ± 16 | 106 ± 12 | 339 ± 31 | 0.9 | 3.2 | |
| 313a | H | CH2…3-methylquinoline | 72 ± 2 | 1160 ± 27 | 111 ± 3 | 3040 ± 252 | 16.1 | 27.4 | |
| 313b | F | CH2…3-methylquinoline | 16 ± 3 | 485 ± 16 | 74 ± 3 | 851 ± 36 | 30.3 | 11.5 | |
| 314a | H | CH2…6-methylquinoline | 190 ± 6 | 845 ± 15 | 140 ± 4 | 1640 ± 58 | 4.4 | 11.7 | |
| 314b | F | CH2…6-methylquinoline | 62 ± 2 | 551 ± 21 | 73 ± 3 | 1040 ± 46 | 8.9 | 14.2 | |
| 315a | H | (CH2)3…2-methyl-1H-1,3-benzodiazole | 23 ± 0.5 | 309 ± 9 | 17 ± 0.7 | 627 ± 12 | 13.4 | 36.9 | |
| 315b | F | (CH2)3…2-methyl-1H-1,3-benzodiazole | 2.5 ± 0.1 | 28 ± 2 | 8.1 ± 0.3 | 74 ± 4 | 11.2 | 9.1 | |
| 316a | H | CH2…2-methylnaphthalene | 43 ± 2 | 903 ± 47 | 32 ± 0.6 | 926 ± 33 | 21.0 | 28.9 | |
| 316b | F | CH2…2-methylnaphthalene | 8.0 ± 0.3 | 312 ± 15 | 30 ± 1 | 588 ± 39 | 39.0 | 19.6 | |
| 317a | H | CH2…1-methylnaphthalene | 114 ± 5 | 336 ± 22 | 406 ± 11 | 83 ± 5 | 2.9 | 0.2 | |
| 317b | F | CH2…1-methylnaphthalene | 31 ± 1 | 243 ± 6 | 312 ± 19 | 257 ± 12 | 7.8 | 0.8 | |
| 318a | H | (CH2)2…1-methylnaphthalene | 92 ± 13 | 462 ± 17 | 42 ± 0.9 | 578 ± 17 | 5.0 | 13.8 | |
| 318b | F | (CH2)2…1-methylnaphthalene | 7.8 ± 0.2 | 46 ± 1 | 25 ± 0.8 | 119 ± 4 | 5.9 | 4.8 | |
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | R″ | IC50 (nM) DAT [3H]WIN 35428 |
IC50 (nM) 5-HTT [3H]citalopram |
Selectivity 5-HTT/DAT |
|---|---|---|---|---|---|---|---|
| 279 (GBR 12909) | 14.0 ± 0.6 | 82 ± 4 | 5.8 | ||||
| 320 (O-549) | 595 ± 148 | 38 ± 127 | 0.6 | ||||
| 321 (O-526) | F | 24.9 ± 3.23 | 248 ± 72 | 9.9 | |||
| 322a | H | 12.0 ± 0.4 | 232 ± 28 | 19.3 | |||
| 322b | Cl | 65.0 ± 12 | 224 ± 10 | 3.4 | |||
| 322c | Br | 159 ± 56 | 835 ± 142 | 5.2 | |||
| 322d | OCH3 | 255 ± 32 | 340 ± 24 | 1.3 | |||
| 323a | H | 10.6 ± 0.85 | 102 ± 5 | 9.6 | |||
| 323b | F | 19.9 ± 9.5 | 31.9 ± 7.1 | 1.6 | |||
| 323c | Cl | 115 ± 22 | 414 ± 32 | 3.6 | |||
| 323d | Br | 382 ± 167 | 638 ± 71 | 1.7 | |||
| 323e | CH3 | 311 ± 71 | 888 ± 58 | 2.8 | |||
| 324a | H | 15.2 ± 2.8 | 743 ± 6 | 48.9 | |||
| 324b | F | 9.7 ± 0.4 | 198 ± 7 | 20.4 | |||
| 325a | H | 14.5 ± 1.9 | 58 ± 7 | 3.7 | |||
| 325b | F | 13.0 ± 2.5 | 112 ± 4 | 8.6 | |||
| 326a | H | 108 ± 14 | 456 ± 90 | 4.2 | |||
| 326b | F | 13.5 ± 2.6 | 237 ± 53 | 17.5 | |||
| 327a | H | 702 ± 34 | 544 ± 91 | 0.8 | |||
| 327b | F | 126 ± 13 | 761 ± 101 | 6.0 | |||
| 328a | H | H | F | 17.2 ± 4.7 | 1920 ± 233 | 111.6 | |
| 328b | H | H | Cl | 24.7 ± 5.5 | 1610 ± 119 | 65.2 | |
| 328c | H | H | Br | 31.1 ± 2.9 | 1490 ± 319 | 47.9 | |
| 328d | H | Cl | Cl | 85.7 ± 4.7 | 2880 ± 281 | 33.6 | |
| 328e | H | H | OCH3 | 27.8 ± 6.8 | 1240 ± 342 | 44.6 | |
| 328f | Cl | H | F | 52.4 ± 7.8 | 1810 ± 107 | 34.5 | |
| 328g | F | H | F | 14.0 ± 3.3 | 1260 ± 72 | 90.0 | |
| 328h | H | H | CH3 | 23.0 ± 3.7 | 1390 ± 240 | 60.4 | |
| 328i | H | Cl | F | 28.2 ± 3.1 | 2530 ± 50 | 89.7 | |
| 328j | H | H | NO2 | 16.4 ± 3.0 | 1770 ± 305 | 107.9 | |
| 328k | H | H | NH2 | 101 ± 13 | 1570 ± 201 | 15.5 | |
| 329a | Ph | 3-pyridyl | 48.6 ± 8.4 | 680 ± 12.0 | 14.0 | ||
| 329b | Ph | 2-benzo[b]thiophenyl | 172 ± 16.4 | 1540 ± 251 | 8.9 | ||
| 329c | Ph | 2-thienyl | 59.3 ± 5.8 | 1250 ± 87 | 21.1 | ||
| 329d | 2-thienyl | Ph | 27.2 ± 0.1 | 741 ± 108 | 27.2 | ||
| 329e | 2-thienyl | 4-F-Ph | 13.8 ± 3.4 | 1390 ± 243 | 101 | ||
| 329f | 2-thienyl | 3-pyridyl | 58.3 ± 5.7 | 927 ± 34 | 15.9 | ||
| 330a | F | 4-F-Ph | 15.1 ± 2.0 | 75.8 ± 22.1 | 5.0 | ||
| 330b | F | Ph | 41.4 ± 8.0 | 271 ± 18.4 | 6.5 | ||
| 330c | H | Ph | 10.1 ± 1.6 | 231 ± 4.5 | 22.9 | ||
| 330d | H | 4-F-Ph | 10.8 ± 3.2 | 205 ± 13.3 | 19.0 | ||
| 330e | H | 2-thienyl | 9.8 ± 2.4 | 290 ± 63 | 29.6 | ||
| 331a | Ph | H | 4-F-Ph | 6.6 ± 1.4 | 223 ± 32.3 | 33.8 | |
| 331b | Ph | H | 3-pyridyl | 29.9 ± 0.3 | 194 ± 20.1 | 6.5 | |
| 331c | 2-thienyl | H | Ph | 6.0 ± 0.5 | 180 ± 21.6 | 30.0 | |
| 331d | 2-thienyl | F | Ph | 11.7 ± 1.0 | 85.7 ± 2.6 | 7.3 | |
| 332a | H | F | 9.4 ± 2.6 | 585 ± 101 | 62.2 | ||
| 332b | F | H | - | - | - |
Local anesthetics (not usually CNS stimulants)
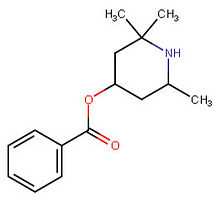
Certain of the local anesthetics still have residual DRI properties,[19] although not normally ones that are easily available. These are expected to be more cardiotoxic than phenyltropanes. For example, dimethocaine has behavioral stimulant effects (and therefore not here listed below) if a dose of it is taken that is 10 times the amount of cocaine. Dimethocaine is equipotent to cocaine in terms of its anesthetic equivalency.[19]
|
|
Note: the above list denotes the intellectual property of copyrighted brands as distinguished from generic names by use of the trademark symbol; these instances being an extenuating differentiation limited to these listings in lieu of writing out "brand name" in long form at every instance.
Analogues for other purposes
Tropanes (Non-ecgonine)
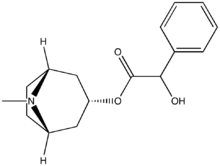
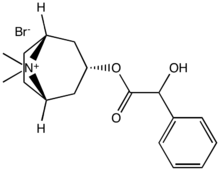
Benzamides
- Procainamide (also an aniline, like dimethocaine)
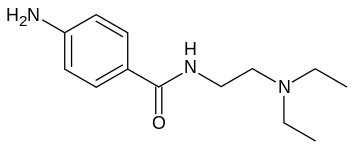
See also
Cocaine-N-oxide: 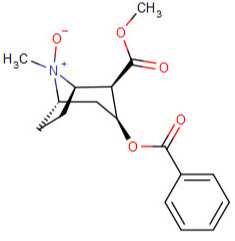 Hydroxytropacocaine:
Hydroxytropacocaine: 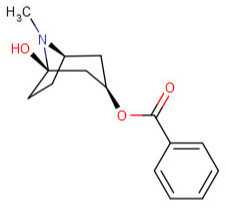 m-Hydroxybenzoylecgonine:
m-Hydroxybenzoylecgonine: 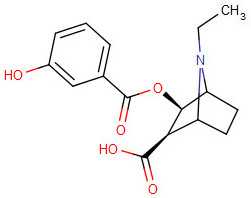

- Coca alkaloids, the ones relating to cocaine biosynthesis include: benzoylecgonine, ecgonidine, ecgonine, hydroxytropacocaine, methylecgonine cinnamate, tropacocaine & truxilline
- Cocaine metabolites (Human), which include: benzoylecgonine (BE), ecgonine methyl ester (EME), ecgonine, norcocaine, p-hydroxycocaine, m-hydroxycocaine, p-hydroxybenzoylecgonine (pOHBE) & m-hydroxybenzoylecgonine
- Dopaminergics
- Federal Analog Act
- Pharmacophore
- Pharmacopoeia
- Pharmacokinetics
Common analogues to prototypical DRAs:
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 1.36 1.37 1.38 Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists. Satendra Singh et al. Chem. Rev. 2000, 100. 925-1024. PubMed; Chemical Reviews (Impact Factor: 45.66). 04/2000; 100(3):925-1024 American Chemical Society; 2000, ISSN: 0009-2665 ChemInform; May, 16th 2000, Volume 31, Issue 20, DOI: 10.1002/chin.200020238. Mirror hotlink.
- ↑ 2.0 2.1 Singh S, Basmadjian GP, Avor K, Pouw B, Seale TW. A convenient synthesis of 2′- or 4′-hydroxycocaine. Synthetic Communications. 1997;27(22):4003-4012.
et. el-Moselhy TF, Avor KS, Basmadjian GP. 2′-substituted analogs of cocaine: synthesis and dopamine transporter binding potencies. Archiv der Pharmazie (Weinheim). 2001 Sep;334(8-9):275-8. PMID 11688137
et. Seale TW, Avor K, Singh S, Hall N, Chan HM, Basmadjian GP. 2′-Substitution of cocaine selectively enhances dopamine and norepinephrine transporter binding. Neuroreport. 10 November 1997;8(16):3571-5. PMID 9427328 - ↑ 3.0 3.1 Smith, R. Martin; Poquette, Michael A.; Smith, Paula J., "Hydroxymethoxybenzoylmethylecgonines: New metabolites of cocaine from human urine." Journal of Analytical Toxicology 1984, 8(1), pp.29-34
- ↑ Gatley SJ, Yu DW, Fowler JS, MacGregor RR, Schlyer DJ, Dewey SL, Wolf AP, Martin T, Shea CE, Volkow ND (March 1994). "Studies with differentially labeled [11C]cocaine, [11C]norcocaine, [11C]benzoylecgonine, and [11C]- and 4′-[18F]fluorococaine to probe the extent to which [11C]cocaine metabolites contribute to PET images of the baboon brain". Journal of Neurochemistry 62 (3): 1154–62. doi:10.1046/j.1471-4159.1994.62031154.x. PMID 8113802.
- ↑ Carroll, F. I.; Lewin, A. H.; Boja, J. W.; Kuhar, M. J. (1992). "Cocaine Receptor: Biochemical Characterization and Structure-Activity Relationships of Cocaine Analogues at Dopamine Transporter". Journal of Medicinal Chemistry 35 (6): 969–981. doi:10.1021/jm00084a001. PMID 1552510.
- ↑ Seale, TW; Avor, K; Singh, S; Hall, N; Chan, HM; Basmadjian, GP (1997). "2′-Substitution of cocaine selectively enhances dopamine and norepinephrine transporter binding". Neuroreport 8 (16): 3571–5. doi:10.1097/00001756-199711100-00030. PMID 9427328.
- ↑ The analgesic properties of some 14-substituted derivatives of codeine and codeinone J. Pharm. Pharmacol., Royal Pharmaceutical Society of Great Britain, 1964, 16, 174—182. doi: 10.1111/j.2042-7158.1964.tb07440.x
- ↑ Benzoylthio-. cocaine, analogue substitution. Synthesis, Properties, and Reactivity of Cocaine Benzoylthio Ester Possessing the Cocaine Absolute Configuration. Shigeki Isomura, Timothy Z. Hoffman, Peter Wirsching, and Kim D. Janda. J. Am. Chem. Soc., 2002, 124 (14), pp 3661–3668 DOI: 10.1021/ja012376y Publication Date (Web): March 14, 2002.
- ↑ "Cocaine Analog Coupled to Disrupted Adenovirus: A Vaccine Strategy to Evoke High-titer Immunity Against Addictive Drugs" PMCID: PMC3048190 doi: 10.1038/mt.2010.280
- ↑ Inhibition of Cocaine Binding to the Human Dopamine Transporter by a Single Chain Anti-Idiotypic Antibody: Its Cloning, Expression and Functional Properties Biochim Biophys Acta. 2003 Jul 30; 1638(3): 257–266. PMCID: PMC3295240 NIHMSID: NIHMS358284
- ↑ Exploring the feasibility of an anti-idiotypic cocaine vaccine: analysis of the specificity of anticocaine antibodies (Ab1) capable of inducing Ab2βanti-idiotypic antibodies Immunology. 2000 May; 100(1): 48–56. PMCID: PMC2326984 doi: 10.1046/j.1365-2567.2000.00004.x
- ↑ Soft drugs— Synthesis and anticholinergic activity of soft phenylsuccinic analogs of methatropine Bioorganic & Medicinal Chemistry: Volume 1, Issue 3, September 1993, Pages 183–187
- ↑ Bemesetron @ U.S. National Library of Medicine's TOXNET: Toxicology Data Network
- ↑ An alkaloid of Phyllanthus discoides (Euphorbiaceae) phyllalbine, a central and peripheral sympathomimetic (Impact Factor: 0.4). 01/1965; 20(4):1033-41
- ↑ Dopamine reuptake transporter (DAT) ``inverse agonism´´ - A novel hypothesis to explain the enigmatic pharmacology of cocaine 2014-12-24 17:08:48 2014-12-25 00:28:27
- ↑ Tanda G, Newman A, Ebbs AL, Tronci V, Green J, Tallarida RJ, Katz JL.Combinations of Cocaine with other Dopamine Uptake Inhibitors: Assessment of Additivity. J Pharmacol Exp Ther. 2009 May 29. PMID 19483071
- ↑ 17.0 17.1 17.2 Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol. 2008 Jan 1;75(1):2-16. doi:10.1016/j.bcp.2007.08.007 PMID 17897630
- ↑ Runyon SP, Carroll FI. Dopamine transporter ligands: recent developments and therapeutic potential. Curr Top Med Chem. 2006;6(17):1825-43. doi:10.2174/156802606778249775 PMID 17017960
- ↑ 19.0 19.1 Wilcox, K.M., Kimmel, H.L., Lindsey, K.P., Votaw, J.R., Goodman, M.M., Howell, L.L. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse, 58: 220-228, 2005. PDF
- ↑ [1] ←Page #969 (45th page of article) §III. ¶1. Final line. Last sentence.
- ↑ [1] ←Page #971 (47th page of article) Figure 30. & Page #973 (49th page of article) Table 28.
- ↑ [1] ←Page #972 (48th page of article) ¶2, Line 10.
- ↑ [1] ←Page #974 (50th page of article) Final ¶ (5th), Second line.
- ↑ [1] ←Page #975 (51st page of article) First ¶, first line.
- ↑ [1] ←Page #975 (51st page of article) First ¶, 4th line.
- ↑ [1] ←Page #973 (49th page of article) §C. & Page #974 (50th page of article) Figure 31 & Page #976 (52nd page of article) Table 29.
- ↑ [1] ←Page #974 (50th page of article) Figure 31 & Page #977 (53rd page of article) Table 30.
- ↑ [1] ←Page #974 (50th page of article) Figure 31 & Page #977 (53rd page of article) Table 30.
- ↑ [1] ←Page #974 (50th page of article) Figure 31 & Page #977 (53rd page of article) Table 30.
- ↑ [1] ←Page #974 (50th page of article) Figure 31 & Page #977 (53rd page of article) Table 30.
- ↑ [1] ←Page #978 (54th page of article) §D & Page #980 (56th page of article) Figure 33 & Page #981 (57th page of article) Table 32.
- ↑ [1] ←Page #978 (54th page of article) §D & Page #980 (56th page of article) Figure 33 & Page #981 (57th page of article) Table 32.
- ↑ [1] ←Page #980 (56th page of article) Scheme 52.
- ↑ [1] ←Page #982 (58th page of article) §G & Page #983 (59th page of article) Figure 36 & Page #984 (60th page of article) Table 35.
- ↑ [1] ←Page #979 (55th page of article) Table 31.
- ↑ [1] ←Page #981 (57th page of article) §E & Page #982 (58th page of article) Table 33.
- ↑ [1] ←Page #982 (58th page of article) 3rd ¶, lines 2, 5 & 6.
- ↑ [1] ←Page #982 (58th page of article) §F, Table 34 & Figure 35.
- ↑ [1] ←Page #984 (60th page of article) §H, Figure 37 & Page #985 (61st page of article) Table 36.
- ↑ [1] ←Page #984 (60th page of article) Scheme 56.
- ↑ [1] ←Page #986 (62nd page of article) §I, Table 37 & Scheme 58
- ↑ [1] ←Page #1,014 (90th page of article) §VIII, A. Figure 59.
- ↑ [1] ←Page #1,016 (92nd page of article) Figure 60.
- ↑ [15] ←Page #31, §3.2. ¶3, 15th & 16th lines, final sentence.
- ↑ [1] ←Page #927 (3rd page of article) second ¶. Lines seven — fifteen.
- ↑ [1] ←Page #987 (63rd page of article) §IV, Figure 39 & Page #988 (64th page of article) Table 38.
- ↑ [1] ←Page #987 (63rd page of article) Figure 40, Page #988 (64th page of article) §B & Page #989 (65th page of article) Table 39.
- ↑ [1] ←Page #987 (63rd page of article) Figure 41, Page #989 (65th page of article) §C & Page #990 (66th page of article) Table 40.
- ↑ [1] ←Page #988 (64th page of article) Figure 42, Page #990 (66th page of article) §2 & Page #992 (68th page of article) Table 41.
- ↑ [1] ←Page #988 (64th page of article) Figure 43, Page #992 (68th page of article) §3 & Table 42.
- ↑ [1] ←Page #993 (69th page of article) §V. ¶2. Fourth line. First sentence.
- ↑ [1] ←Page #993 (69th page of article) §V, Figure 46 & Table 43.
- ↑ [1] ←Page #995 (71st page of article) Figure 47 & Page #997 (73rd page of article) Table 44.
- ↑ [1] ←Page #997 (73rd page of article) §C, Page #998 (74th page of article) Figure 48 & Page #1,000 Table 45.
- ↑ [1] ←Page #1,000 (76th page of article) §D, Page #1,001 (77th page of article) Figure 49 & Page #1,005 (81st page of article) Table 46.
External links
| Wikimedia Commons has media related to Cocaine analogs. |
- U. S. Provisional Patent Application listing examples of compounds which are tropanes for prospective use in research
- Article on cocaine analogue research
- List of tropanyl type molecules and their CAS Registry Numbers
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||
| ||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||