Lisinopril
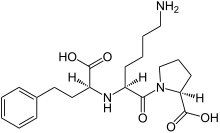 | |
 Chemical structure of lisinopril | |
| Systematic (IUPAC) name | |
|---|---|
| N2-[(1S)-1-carboxy-3-phenylpropyl]-L-lysyl-L-proline | |
| Clinical data | |
| Trade names | Prinivil, Tensopril, Zestril, Hipril |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a692051 |
| |
| |
| Oral | |
| Pharmacokinetic data | |
| Bioavailability | approx. 25%, but wide range between individuals (6 to 60%) |
| Protein binding | 0 |
| Metabolism | None |
| Half-life | 12 hours |
| Excretion | Eliminated unchanged in urine |
| Identifiers | |
| 83915-83-7 | |
| C09AA03 | |
| PubChem | CID 5362119 |
| DrugBank |
APRD00560 |
| ChemSpider |
4514933 |
| UNII |
7Q3P4BS2FD |
| KEGG |
D00362 |
| ChEBI |
CHEBI:43755 |
| ChEMBL |
CHEMBL1237 |
| Synonyms | (2S)-1-[(2S)-6-amino-2-{[(1S)-1-carboxy-3-phenylpropyl]amino}hexanoyl]pyrrolidine-2-carboxylic acid |
| PDB ligand ID | LPR (PDBe, RCSB PDB) |
| Chemical data | |
| Formula | C21H31N3O5 |
| 405.488 g/mol | |
|
SMILES
| |
| |
| | |
Lisinopril (/laɪˈsɪnəprɪl/ ly-SIN-ə-pril) is a drug of the angiotensin-converting enzyme (ACE) inhibitor class used primarily in treatment of hypertension, congestive heart failure, and heart attacks, and in preventing renal and retinal complications of diabetes. Its indications, contraindications, and side effects are as those for all ACE inhibitors.
Lisinopril was the third ACE inhibitor (after captopril and enalapril) and was introduced into therapy in the early 1990s.[2] A number of properties distinguish it from other ACE inhibitors: It is hydrophilic, has a long half-life and tissue penetration, and is not metabolized by the liver.
Medical uses
Lisinopril is typically used for the treatment of hypertension, congestive heart failure, acute myocardial infarction, and diabetic nephropathy.[1]
Special populations
The dose must be adjusted in those with poor kidney function.[3]
Contraindications
- History of angioedema (hereditary or idiopathic)[4]
- Hypersensitivity reaction to lisinopril or any of other components found in its formulation[5]
- Concomitant use of aliskiren-containing drugs in patients with diabetes[6]
Combination of aliskiren and lisinopril is contraindicated in patients with diabetes, and should be avoided in those with creatinine clearance less than 60ml/min. This combination may heighten the hyperkalemic, hypotensive, and nephrotoxic effects of lisinopril (and other ACE inhibitors). Therefore, serum potassium, blood pressure, and serum creatinine should be carefully monitored for all patients on this combination.
Adverse effects
Side effects, some of which are serious and require immediate medical attention, may include:[7]
- Chills, signs of infection
- Dark urine (melanuria)
- Decreased urination (oliguria)
- Difficulty swallowing or breathing (signs of angioedema), allergic reaction (anaphylaxis)
- Hoarseness
- Itching
- Yellowing of skin or eyes (jaundice)
- Abdominal pain, bloating, vomiting
- Chest pain or tightness, dizziness, lightheadedness, fainting
- Dry cough
- Fever
- Joint pain
- Rash
- Diarrhea, nausea
- Drowsiness, headache, tiredness
- Muscle cramps
- Dry mouth
- Serious (possibly fatal) liver problems
- Impotence[8]
- Dizziness
- Faintness [9]
Lisinopril causes the kidneys to retain potassium, which may lead to hyperkalemia. From a study on eHealthMe of more than 1,000 patients with hyperkalemia when using it, the condition may happen more in older male users.[10]
A rare but severe allergic reaction that affects the bowel wall and secondarily causes abdominal pain can occur. This "angioedema" reaction is very rare and must be given immediate medical attention.[11]
Pregnancy and breastfeeding
Lisinopril has been assigned to pregnancy category D by the FDA for use during the second and third trimesters and to category C during the first trimester. Animal and human data have revealed evidence of embryolethality and teratogenicity associated with ACE inhibitors. No controlled data in human pregnancy are available. Congenital malformations have been reported with the use of ACE inhibitors during the first trimester of pregnancy, while fetal and neonatal toxicity, death, and congenital anomalies have been reported with their use during the second and third trimesters of pregnancy. If the patient becomes pregnant, lisinopril should be discontinued as soon as possible; it is considered contraindicated during pregnancy.
No data exist on the excretion of lisinopril into human milk. The manufacturer recommends, due to the potential for serious adverse reactions in nursing infants, a decision should be made to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. [12]

Pharmacology
Lisinopril is the lysine-analog of enalapril. Unlike other ACE inhibitors, it is not a prodrug and is excreted unchanged in the urine. In cases of overdosage, it can be removed from circulation by dialysis.[13]
Pharmacokinetics and metabolism
For adult patients, following oral administration of lisinopril, peak serum concentrations occur within about seven hours, although a trend to a small delay in time taken to reach peak serum concentrations in acute myocardial infarction patients was seen. Declining serum concentrations exhibit a prolonged terminal phase that does not contribute to drug accumulation. This terminal phase probably represents saturable binding to ACE and is not proportional to dose. Lisinopril does not appear to be bound to other serum proteins.
Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine. Based on urinary recovery, the mean extent of its absorption is approximately 25%, with large intersubject variability (6–60%) at all doses tested (5–80 mg). Lisinopril absorption is not influenced by the presence of food in the gastrointestinal tract. In patients with stable NYHA class II-IV congestive heart failure, its absolute bioavailability is reduced to about 16%, and the volume of distribution appears to be slightly smaller than that in normal subjects.
The oral bioavailability of lisinopril in patients with acute myocardial infarction is similar to that in healthy volunteers. Upon multiple dosing, it exhibits an effective half-life of accumulation of 12 hours.
Impaired renal function decreases elimination of lisinopril, which is excreted principally through the kidneys, but this decrease becomes clinically important only when the glomerular filtration rate is below 30 ml/min. Above this rate, the elimination half-life is little-changed. With greater impairment, however, peak and trough levels increase, time to peak concentration increases, and time to attain steady state is prolonged. Older patients, on average, have higher (around doubled) blood levels and area under the plasma concentration time curve than younger patients.
Brand names
Lisinopril was developed by Merck & Co., and is marketed worldwide as Prinivil or Tensopril, and by AstraZeneca as Zestril. In India, it is marketed by Micro Labs as Hipril. In the United States, a generic version is available. Like other ACE inhibitors, it is a synthetic functional and structural analog of a peptide derived from the venom of the jararaca, a Brazilian pit viper (Bothrops jararaca).[14] Lisinopril can also be used in conjunction with the diuretic hydrochlorothiazide, and drugs that combine these two medications are available under the brand names Prinzide and Zestoretic.
See also
References
- ↑ 1.0 1.1 "Lisinopril". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ↑ Patchett A, Harris E, Tristram E, Wyvratt M, Wu M, Taub D, Peterson E, Ikeler T, ten Broeke J, Payne L, Ondeyka D, Thorsett E, Greenlee W, Lohr N, Hoffsommer R, Joshua H, Ruyle W, Rothrock J, Aster S, Maycock A, Robinson F, Hirschmann R, Sweet C, Ulm E, Gross D, Vassil T, Stone C (1980). "A new class of angiotensin-converting enzyme inhibitors". Nature 288 (5788): 280–3. doi:10.1038/288280a0. PMID 6253826.
- ↑ "Lisinopril Dosage, Interactions, Side Effects, How to Use". HealthDigest.org.
- ↑ Product Information: ZESTRIL(R) oral tablet, lisinopril oral tablet. AstraZeneca Pharmaceuticals LP, Wilmington, DE, 2005.
- ↑ Product Information: ZESTRIL(R) oral tablet, lisinopril oral tablet. AstraZeneca Pharmaceuticals LP, Wilmington, DE, 2005.
- ↑ Product Information: ZESTRIL(R) oral tablet, lisinopril oral tablet. AstraZeneca Pharmaceuticals LP, Wilmington, DE, 2005.
- ↑ "Lisinopril Oral : Uses, Side Effects, Interactions, Pictures, Warnings & Dosing". WebMD. Retrieved 20 April 2010.
- ↑ http://www.lisinopril.com/index.php?id=lisinopril_sideeffects
- ↑ http://www.webmd.com/drugs/2/drug-6873/lisinopril-oral/details/list-sideeffects
- ↑ "Hyperkalemia in the use of Lisinopril, who, when, how?". eHealthMe. Retrieved 31 August 2010.
- ↑ http://www.ccjm.org/content/78/5/297.full
- ↑ "Lisinopril". US Marketed Drugs www.drugsdb.eu.
- ↑ AstraZeneca. "ZESTRIL (lisinopril) product insert" (PDF). accessdata.fda.gov. Retrieved 15 October 2011.
- ↑ Patlak M (March 2004). "From viper's venom to drug design: treating hypertension". FASEB J. 18 (3): 421. doi:10.1096/fj.03-1398bkt. PMID 15003987.
Further reading
- Fogari R, Zoppi A, Corradi L, Lazzari P, Mugellini A, Lusardi P (November 1998). "Comparative effects of lisinopril and losartan on insulin sensitivity in the treatment of non diabetic hypertensive patients". Br J Clin Pharmacol 46 (5): 467–71. doi:10.1046/j.1365-2125.1998.00811.x. PMC 1873694. PMID 9833600.
- Bussien JP, Waeber B, Nussberger J, Gomez HJ, Brunner HR (1985). "Once-daily lisinopril in hypertensive patients: Effect on blood pressure and the renin-angiotensin system". Curr Therap Res 37: 342–51.
External links
- Prescribing information for Zestril
- U.S. National Library of Medicine: Drug Information Portal – Lisinopril
- Lisinopril.com – Lisinopril information
| ||||||||||||||||||||||||||||||||