Lipid droplet
Lipid droplets, also referred to as lipid bodies, oil bodies or adiposomes,[1] are lipid-rich cellular organelles that regulate the storage and hydrolysis of neutral lipids and are found largely in the adipose tissue.[2] They also serve as a reservoir for cholesterol and acyl-glycerols for membrane formation and maintenance. Lipid droplets are found in all eukaryotic organisms and store a large portion of lipids in mammalian adipocytes. Initially, these lipid droplets were considered to merely serve as fat depots, but since the discovery in the nineties of proteins in the lipid droplet coat that regulate lipid droplet dynamics and lipid metabolism, lipid droplets are seen as highly dynamic organelles that play a very important role in the regulation of intracellular lipid storage and lipid metabolism. The role of lipid droplets outside of lipid and cholesterol storage has recently begun to be elucidated and includes a close association to inflammatory responses through the synthesis and metabolism of eicosanoids and to metabolic disorders such as obesity, cancer,[3][4] and atherosclerosis.[5] In non-adipocytes, lipid droplets are known to play a role in protection from lipotoxicity by storage of fatty acids in the form of neutral triacylglycerol, which consists of three fatty acids bound to glycerol. Alternatively, fatty acids can be converted to lipid intermediates like diacylglycerol (DAG), ceramides and fatty acyl-CoAs. These lipid intermediates can impair insulin signaling, which is referred to as lipid-induced insulin resistance and lipotoxicity.[6] Lipid droplets also serve as platforms for protein binding and degradation. Finally, lipid droplets are known to be exploited by pathogens such as the hepatitis C virus, the dengue virus and chlamydia trachomatis among others.[7]
Structure
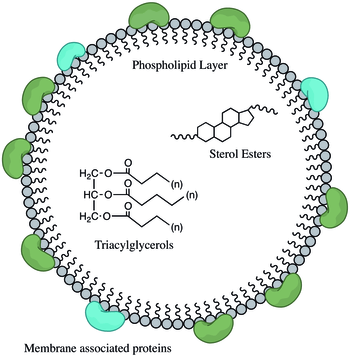
Lipid droplets are composed of a neutral lipid core consisting mainly of triacylglycerols (TAGs) and cholesteryl esters surrounded by a phospholipid monolayer.[2] The surface of lipid droplets is decorated by a number of proteins which are involved in the regulation of lipid metabolism.[2] The first and best-characterized family of lipid droplet coat proteins is the perilipin protein family, consisting of five proteins. These include perilipin 1 perilipin/ PLIN1, perilipin 2 (PLIN2/ ADRP),[8] perilipin 3 (PLIN3/ TIP47), perilipin 4 (PLIN4/ S3-12) and perilipin 5 (PLIN5/ OXPAT/ LSDP5/ MLDP).[9][10][11] Proteomics studies have elucidated the association of many other families of proteins to the lipid surface including proteins involved in membrane trafficking, vesicle docking, endocytosis and exocytosis.[12] Analysis of the lipid composition of lipid droplets has revealed the presence of a diverse set of phospholipid species;[13] phosphatidylcholine and phosphatidylethanolamine are the most abundant, followed by phosphatidylinositol.
Lipid droplets vary greatly in size, ranging from 20-40 nm to 100 um.[14] In adipocytes, lipid bodies tend to be larger and they may compose the majority of the cell, while in other cells they may only be induced under certain conditions and are considerably smaller in size.
Formation
Although the exact mechanism of formation of lipid droplets is still unknown, it is proposed that they bud off the membrane of the endoplasmic reticulum as TAGs are collected between the two layers of its phospholipid membrane. Lipid droplet growth can consequently happen through direct diffusion of fatty acids, endocytosis of sterols or by fusion of smaller lipid droplets through the aid of SNARE proteins.[14] Lipid droplets have also been observed to be created by the fission of existing lipid droplets, though this is thought to be less common than de novo formation. [15]
The formation of lipid droplets from the endoplasmic reticulum begins with the synthesis of the neutral lipids to be transported. The manufacture of TAGs from diacylglycerol (by the addition of a fatty acyl chain) is catalyzed by the DGAT proteins, though the extent to which these and other proteins are required depends on cell type.[16] Neither DGAT1 nor DGAT2 is singularly essential for TAG synthesis or droplet formation, though mammalian cells lacking both cannot form lipid droplets and have severely stunted TAG synthesis. DGAT1, which seems to prefer exogenous fatty acid substrates, is not essential for life; DGAT2, which seems to prefer endogenously synthesized fatty acids, is.[15]
In non-adipocytes, lipid storage, lipid droplet synthesis and lipid droplet growth can be induced by various stimuli including growth factors, long-chain unsaturated fatty acids (including oleic acid and arachidonic acid), oxidative stress and inflammatory stimuli such bacterial lipopolysaccharides, various microbial pathogens, platelet-activating factor, eicosanoids, and cytokines.[17]
Gallery
-
Lipid droplets in murine microglia -

Oil bodies in Nardia scalaris, a leafy liverwort
See also
References
- ↑ Martin, Sally; Parton, Robert G. (8 March 2006). "Lipid droplets: a unified view of a dynamic organelle". Nature Reviews Molecular Cell Biology 7 (5): 373–378. doi:10.1038/nrm1912.
- ↑ 2.0 2.1 2.2 Mobilization and cellular uptake of stored fats and triacylglycerol (with Animation)
- ↑ Bozza, PT; Viola, JP (Apr–Jun 2010). "Lipid droplets in inflammation, cancer". Prostaglandins, leukotrienes, and essential fatty acids 82 (4-6): 243–50. doi:10.1016/j.plefa.2010.02.005. PMID 20206487.
- ↑ Melo, Rossana C. N.; Dvorak, Ann M.; Chitnis, Chetan E. (5 July 2012). "Lipid Body–Phagosome Interaction in Macrophages during Infectious Diseases: Host Defense or Pathogen Survival Strategy?". PLoS Pathogens 8 (7): e1002729. doi:10.1371/journal.ppat.1002729.
- ↑ Greenberg, Andrew S.; Coleman, Rosalind A.; Kraemer, Fredric B.; McManaman, James L.; Obin, Martin S.; Puri, Vishwajeet; Yan, Qing-Wu; Miyoshi, Hideaki; Mashek, Douglas G. (1 June 2011). "The role of lipid droplets in metabolic disease in rodents and humans". Journal of Clinical Investigation 121 (6): 2102–2110. doi:10.1172/JCI46069.
- ↑ Bosma M, Kersten S, Hesselink MKC, and Schrauwen P. Re-evaluating lipotoxic triggers in skeletal muscle: Relating intramyocellular lipid metabolism to insulin sensitivity. Prog Lipid Res 2012; 51: 36-49|doi=10.1016/j.plipres.2011.11.003
- ↑ Suzuki, M.; Shinohara, Y.; Ohsaki, Y.; Fujimoto, T. (15 August 2011). "Lipid droplets: size matters". Journal of Electron Microscopy 60 (supplement 1): S101–S116. doi:10.1093/jmicro/dfr016.
- ↑ Bosma M, Hesselink MKC, Sparks LM, Timmers S, Ferraz MJ, Mattijssen F, van Beurden D, Schaart G, de Baets MH, Verheyen FK, Kersten S, and Schrauwen P. Perilipin 2 improves insulin sensitivity in skeletal muscle despite elevated intramuscular lipid levels. Diabetes 2012; 61: 2679-2690. doi=10.2337/db11-1402
- ↑ Martin, S; Parton, RG (Apr 2005). "Caveolin, cholesterol, and lipid bodies.". Seminars in cell & developmental biology 16 (2): 163–74. doi:10.1016/j.semcdb.2005.01.007. PMID 15797827.
- ↑ Brasaemle, D. L. (25 August 2007). "Thematic review series: Adipocyte Biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis". The Journal of Lipid Research 48 (12): 2547–2559. doi:10.1194/jlr.R700014-JLR200. PMID 17878492.
- ↑ Kimmel, AR; Brasaemle, DL; McAndrews-Hill, M; Sztalryd, C; Londos, C (Mar 2010). "Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins.". Journal of lipid research 51 (3): 468–71. doi:10.1194/jlr.R000034. PMC 2817576. PMID 19638644.
- ↑ Goodman, JM (Oct 17, 2008). "The gregarious lipid droplet.". The Journal of Biological Chemistry 283 (42): 28005–9. doi:10.1074/jbc.R800042200. PMC 2568941. PMID 18611863.
- ↑ Bartz, R.; Li, W.-H.; Venables, B.; Zehmer, J. K.; Roth, M. R.; Welti, R.; Anderson, R. G. W.; Liu, P.; Chapman, K. D. (22 January 2007). "Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic,". The Journal of Lipid Research 48 (4): 837–847. doi:10.1194/jlr.M600413-JLR200.
- ↑ 14.0 14.1 Guo, Y; Cordes, KR; Farese RV, Jr; Walther, TC (Mar 15, 2009). "Lipid droplets at a glance.". Journal of Cell Science 122 (Pt 6): 749–52. doi:10.1242/jcs.037630. PMC 2714424. PMID 19261844.
- ↑ 15.0 15.1 Wilfling, Florian; Haas, J.; Walther, T.; Farese, R. (August 2014). "Lipid droplet biogenesis". Current Opinion in Cell Biology 29: 39–45. doi:10.1016/j.ceb.2014.03.008.
- ↑ Harris, Charles; et. al. (April 2011). "DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes". Journal of Lipid Research 52 (4): 657–667. doi:10.1194/jlr.M013003.
- ↑ Melo, R. C. N.; D'Avila, H.; Wan, H.-C.; Bozza, P. T.; Dvorak, A. M.; Weller, P. F. (23 March 2011). "Lipid Bodies in Inflammatory Cells: Structure, Function, and Current Imaging Techniques". Journal of Histochemistry & Cytochemistry 59 (5): 540–556. doi:10.1369/0022155411404073.