Leucines
The leucines are primarily the four isomeric amino acids: leucine, isoleucine, tert-leucine and norleucine. Being compared with the four butanols, they could be classified as butyl-substituted glycines; they represent all four possible variations.
Leucine and isoleucine belong to the proteinogenic amino acids; the others are non-natural.
Including the stereoisomers, six further isomers could be added: D-leucine, D-isoleucine, L-allo-isoleucine, D-allo-isoleucine, D-tert-leucine and D-norleucine.
Cycloleucine could be classified as a cyclic derivate of norleucine. With a cyclopentane-ring, it has two hydrogen atoms fewer and thus is not an isomer. The α-carbon atom is not a stereocenter.
| Leucines | |||||
| Name | L-Leucine | L-Isoleucine | L-tert-Leucine (Terleucine) | L-Norleucine | Cycloleucine |
| Other names | 2-Amino-4-methylpentanoic acid, Isobutylglycine |
2-Amino-3-methylpentanoic acid, sec-Butylglycine |
2-Amino-3,3-dimethylbutanoic acid, tert-Butylglycine |
2-Amino-hexanoic acid, n-Butylglycine |
1-Amino-cyclopentane-1-carboxylic acid |
| Structure | 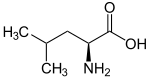 | 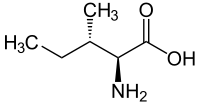 | 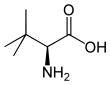 | 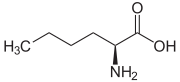 | 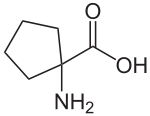 |
| CAS-number | 61-90-5 | 73-32-5 | 20859-02-3 | 327-57-1 | 52-52-8 |
| PubChem | PubChem 6106 | PubChem 791 | PubChem 164608 | PubChem 21236 | PubChem 2901 |
| Molecular formula | C6H13NO2 | C6H11NO2 | |||
| Molar mass | 131.18 g/mol | 129.16 g/mol | |||