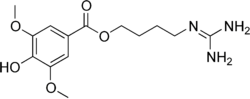
Leonurine
 | |
| Names | |
|---|---|
| IUPAC name
4-Hydroxy-3,5-dimethoxybenzoic acid 4-guanidinobutyl ester | |
| Identifiers | |
| 24697-74-3 | |
| ChemSpider | 141828 |
| |
| Jmol-3D images | Image |
| KEGG | C16985 |
| PubChem | 161464 |
| |
| Properties | |
| Molecular formula |
C14H21N3O5 |
| Molar mass | 311.33 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Leonurine is one of the chemical constituents of the South African plant Leonotis leonurus (Lion's tail). It is a mildly psychoactive alkaloid found in species Leonotis nepetifolia, Leonotis artemisia, Leonurus cardiaca (Motherwort) as well as other plants of family Lamiaceae. Leonurine is easily extracted into water, as well as from the essential oil of Leonurus sibiricus.[1]
Chemical Synthesis
- Trimethylgallic acid (Eudesmic acid) is cleaved with 20% sulphuric acid.
- Syringic acid is protected with ethylchloroformate.
- 4-carboethoxysyringic acid reacted with thionyl chloride SOCl2.
- residue reacted with THF yielding 4-carboethoxysyringic acid 4-chloro-1-butyl ester
- Gabriel synthesis (Potassium pthalimide) followed by hydrazinolysis (Ing–Manske procedure).
- Last step is reaction of the amine with S-Methylisothiourea hemisulfate salt,[2]
yielding 4-Guanidino-1-butanol Syringate.

Leonurine synthesis.[1]
Traditional uses
Primary uses include treatment of:
- Respiratory tract infections caused by Streptococcus pneumoniae and group A β-hemolytic streptococci.
- Otitis media due to S. pneumoniae, Hemophilus influenzae, Moraxella catarrhalis, staphylococci, streptococci, and N. catarrhalis.
- GU infections (including acute prostatitis) due to Escherichia coli, Proteus mirabilis or Klebsiella species.
- Bone infections caused by P. mirabilis or staphylococci.
- Skin and skin structure infections due to staphylococci and streptococci.
References
- ↑ 1.0 1.1 "The Leonurine and its preparation". An Hui New Star Pharmaceutical Development Co. 2008. Retrieved 2008-08-28.
- ↑ "S-Methylisothiourea sulfate structure.". ChemSpider (RSC). 2014. Retrieved 2014-07-09.
Further reading
- Cheng KF, Yip CS, Yeung HW, Kong YC (May 1979). "Leonurine, an improved synthesis". Experientia 35 (5): 571–2. doi:10.1007/BF01960323. PMID 446644.