Lemieux–Johnson oxidation
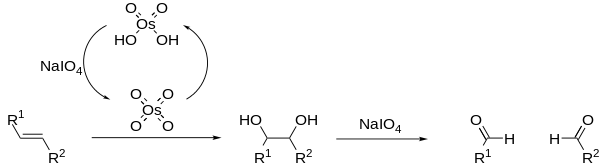
The Lemieux–Johnson oxidation is a chemical reaction in which an olefin undergoes oxidative cleavage to form two aldehyde or ketone units. The reaction is named after its inventors, R. U. Lemieux and W. S. Johnson, who published it in 1956.[1] The reaction proceeds in a two step manner, beginning with dihydroxylation of the alkene by osmium tetroxide, followed by oxidative cleavage by periodate. Excess periodate is used to regenerate the osmium tetroxide, allowing it to be used in catalytic amounts. The Lemieux–Johnson reaction ceases at the aldehyde stage of oxidation and therefore produces the same results as ozonolysis.
The classical Lemieux–Johnson oxidation often generates many side products, resulting in low reaction yields; however the addition of non-nucleophilic bases, such as 2,6-lutidine, can improve on this.[2] OsO4 may be replaced with an number of other Osmium compounds.[3][4] Periodate may also be replaced with other oxidising agents, such as oxone.[5]
See also
References
- ↑ Pappo, R.; Allen, D. S., Jr.; Lemieux, R. U.; Johnson, W. S. (1956). "Osmium Tetroxide-Catalyzed Periodate Oxidation of Olefinic Bonds.". J. Org. Chem. 21 (4): 478–479. doi:10.1021/jo01110a606.
- ↑ Hua, Z.; Yu, W.; Jin, Z. (2004). "An Improved Procedure for the Oxidative Cleavage of Olefins by OsO4-NaIO4". Org. Lett. 6 (19): 3217. doi:10.1021/ol0400342.
- ↑ Whitehead, Daniel C.; Travis, Benjamin R.; Borhan, Babak. "The OsO4-mediated oxidative cleavage of olefins catalyzed by alternative osmium sources". Tetrahedron Letters 47 (22): 3797–3800. doi:10.1016/j.tetlet.2006.03.087.
- ↑ Kim, Seyoung; Chung, Jooyoung; Kim, B. Moon. "Recycling of osmium catalyst in oxidative olefin cleavage: a chemoentrapment approach". Tetrahedron Letters 52 (12): 1363–1367. doi:10.1016/j.tetlet.2011.01.065.
- ↑ Travis, Benjamin R.; Narayan, Radha S.; Borhan, Babak. "Osmium Tetroxide-Promoted Catalytic Oxidative Cleavage of Olefins: An Organometallic Ozonolysis". Journal of the American Chemical Society 124 (15): 3824–3825. doi:10.1021/ja017295g.