Laurolactam
 | |
| Names | |
|---|---|
| IUPAC name
azacyclotridecan-2-one | |
| Other names
Dodecalactam | |
| Identifiers | |
| 947-04-6 | |
| ChemSpider | 13099 |
| |
| Jmol-3D images | Image |
| PubChem | 13690 |
| |
| Properties | |
| Molecular formula |
C12H23NO |
| Molar mass | 197.32 g·mol−1 |
| Appearance | colourless solid |
| Melting point | 152.5 °C (306.5 °F; 425.6 K)[1] |
| Boiling point | 314.9±10 °C |
| 0.03 wt% | |
| Hazards | |
| Flash point | 192 °C |
| 320 to 330 °C | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Laurolactam is an organic compound with the molecular formula (CH2)11C(O)NH. This colorless solid is classified as a lactam. Laurolactam is mainly used as a monomer in engineering plastics, such as nylon-12 and copolyamides.
Synthesis
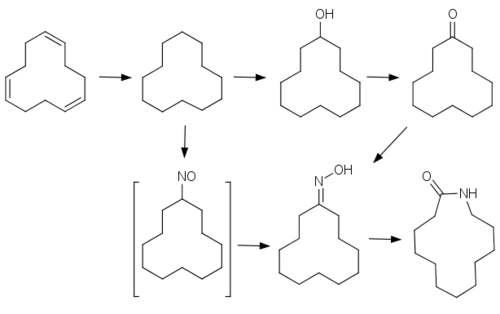
Although formally a derivative of 12-aminododecanoic acid]], it is made quite differently starting from cyclododecane. Cylcododecatriene is hydrogenated to the unsaturated alkane, cyclododecane. In the process adopted by Evonik Industries, the cycloalkane is oxidized to the ketone derivative with a boric acid catalyst. The cyclic ketone is converted to the oxime functional group by condensation with hydroxylamine. Under strong acidic conditions and elevated temperatures (115 °C), the oxime converts via a Beckmann rearrangement laurolactam.
The procedure used by Arkema begins with treatment of cyclododecane with nitrosyl chloride, resulting in the unstable nitrosocyclododecane, which undergoes tautomeric transformation to the cyclododecanone oxime, which again is amenable to Beckmann rearrangement.[2]
Uses
Ring opening polymerization is used to convert the monomer to nylon-12. The reaction can be brought about with cationic or anionic initiators or with water . Cationic polymerization with acid is believed to involve the initial O-protonation. Nucleophilic attack by the monomer on the reactive protonated nitrogen, followed by successive ring-opening acylation of the primary amine results in the formation of the polyamide.[3]
References
- ↑ Bradley, Jean-Claude; Williams, Antony; Lang, Andrew (2014): Jean-Claude Bradley Open Melting Point Dataset. figshare. doi:10.6084/m9.figshare.1031637
- ↑ Schiffer, T.; Oenbrink, G. "Cyclododecanol, Cyclododecanone, and Laurolactam" in Ullman’s Encyclopedia of Industrial Chemistry: Wiley-VCH, 2009. doi:10.1002/14356007.a08_201.pub2
- ↑ Stevens, M. P. Polymer Chemistry : An Introduction, Oxford University Press: New York, 1999.