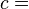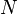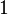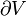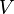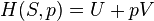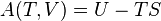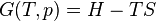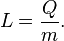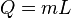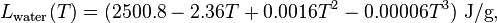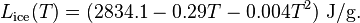Latent heat
Latent heat is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process. An example is a state of matter change, meaning a phase transition, such as ice melting or water boiling.[1][2] The term was introduced around 1762 by Scottish chemist Joseph Black. It is derived from the Latin latere (to lie hidden). Black used the term in the context of calorimetry where a heat transfer caused a volume change while the thermodynamic system's temperature was constant.
In contrast to latent heat, sensible heat involves an energy transfer that results in a temperature change of the system.
| Thermodynamics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
The classical Carnot heat engine | ||||||||||||
|
Branches |
||||||||||||
|
||||||||||||
| Book:Thermodynamics | ||||||||||||
Usage
The terms ″sensible heat″ and ″latent heat″ are not special forms of energy; instead they measure two kinds of change in a material or in a thermodynamic system. ″Sensible heat″ measures change in a body's internal energy that may be ″sensed″ with a thermometer. ″Latent heat″ measures change in internal energy that seems hidden from a thermometer – the temperature reading doesn't change. Heat is energy in the process of transferring between a system and its surroundings, other than as work or by transfer of matter.
Both sensible and latent heats are observed in many processes of transport of energy in nature. Latent heat is associated with the phase changes of atmospheric water vapor, mostly vaporization and condensation, whereas sensible heat is energy transferred that affects the temperature of the atmosphere.
The original usage of the term, as introduced by Black, was applied to systems that were intentionally held at constant temperature. Such usage referred to latent heat of expansion and several other related latent heats. These latent heats are defined independently of the conceptual framework of thermodynamics.[3]
When a body is heated at constant temperature by thermal radiation in a microwave field for example, it may expand by an amount described by its latent heat with respect to volume or latent heat of expansion, or increase its pressure by an amount described by its latent heat with respect to pressure.[4]
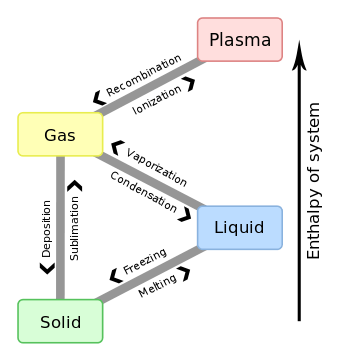
Two common forms of latent heat are latent heat of fusion (melting) and latent heat of vaporization (boiling). These names describe the direction of energy flow when changing from one phase to the next: from solid to liquid, and liquid to gas.
In both cases the change is endothermic, meaning that the system absorbs energy. If the change is exothermic, then energy is released. For example, when water evaporates energy is transferred from a water molecule to an air molecule that contains less water vapor than its surroundings. Because energy is required for the water molecule to overcome the forces of attraction between water particles, the transition from water to vapor requires an input of energy and causes a temperature drop in the water molecule's surroundings.
If the vapor then condenses to a liquid on a surface, then the vapor's latent energy absorbed during evaporation is released as the liquid's sensible heat onto the surface.
The large value of the enthalpy of condensation of water vapor is the reason that steam is a far more effective heating medium than boiling water, and is more hazardous.
Meteorology
In meteorology, latent heat flux is the flux of heat from the Earth's surface to the atmosphere that is associated with evaporation or transpiration of water at the surface and subsequent condensation of water vapor in the troposphere. It is an important component of Earth's surface energy budget. Latent heat flux has been commonly measured with the Bowen ratio technique, or more recently since the mid-1900s by the eddy covariance method.
History
The English word latent comes from Latin latēns, meaning lying hidden.[5][6] The term latent heat was introduced into calorimetry around 1750 when Joseph Black, commissioned by producers of Scotch whisky in search of ideal quantities of fuel and water for their distilling process,[7] to studying system changes, such as of volume and pressure, when the thermodynamic system was held at constant temperature in a thermal bath. James Prescott Joule characterised latent energy as the energy of interaction in a given configuration of particles, i.e. a form of potential energy, and the sensible heat as an energy that was indicated by the thermometer,[8] relating the latter to thermal energy.
Specific latent heat
A specific latent heat (L) expresses the amount of energy in the form of heat (Q) required to completely effect a phase change of a unit of mass (m), usually 1kg, of a substance as an intensive property:
Intensive properties are material characteristics and are not dependent on the size or extent of the sample. Commonly quoted and tabulated in the literature are the specific latent heat of fusion and the specific latent heat of vaporization for many substances.
From this definition, the latent heat for a given mass of a substance is calculated by
where:
- Q is the amount of energy released or absorbed during the change of phase of the substance (in kJ or in BTU),
- m is the mass of the substance (in kg or in lb), and
- L is the specific latent heat for a particular substance (kJ-kgm−1 or in BTU-lbm−1), either Lf for fusion, or Lv for vaporization.
Table of latent heats
The following table shows the latent heats and change of phase temperatures of some common fluids and gases.
| Substance | Latent Heat Fusion kJ/kg |
Melting Point °C |
Latent Heat Vaporization kJ/kg |
Boiling Point °C |
|---|---|---|---|---|
| Alcohol, ethyl | 108 | −114 | 855 | 78.3 |
| Ammonia | 332.17 | −77.74 | 1369 | −33.34 |
| Carbon dioxide | 184 | −78 | 574 | −57 |
| Helium | 21 | −268.93 | ||
| Hydrogen(2) | 58 | −259 | 455 | −253 |
| Lead[9] | 23.0 | 327.5 | 871 | 1750 |
| Nitrogen | 25.7 | −210 | 200 | −196 |
| Oxygen | 13.9 | −219 | 213 | −183 |
| Refrigerant R134a | −101 | 215.9 | −26.6 | |
| Refrigerant R152a | −116 | 326.5 | -25 | |
| Toluene | 72.1 | −93 | 351 | 110.6 |
| Turpentine | 293 | |||
| Water | 336 | 0 | 2260 | 100 |
Latent heat for condensation of water
The latent heat of condensation of water in the temperature range from −25 °C to 40 °C is approximated by the following empirical cubic function:
where the temperature 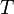 is taken to be the numerical value in °C.
is taken to be the numerical value in °C.
For sublimation and deposition from and into ice, the latent heat is almost constant in the temperature range from −40 °C to 0 °C and can be approximated by the following empirical quadratic function:
See also
- Bowen ratio
- Eddy covariance flux (eddy correlation, eddy flux)
- Sublimation (physics)
- Specific heat capacity
- Enthalpy of fusion
- Enthalpy of vaporization
References
- ↑ Perrot, Pierre (1998). A to Z of Thermodynamics. Oxford University Press. ISBN 0-19-856552-6.
- ↑ Clark, John, O.E. (2004). The Essential Dictionary of Science. Barnes & Noble Books. ISBN 0-7607-4616-8.
- ↑ Bryan, G.H. (1907). Thermodynamics. An Introductory Treatise dealing mainly with First Principles and their Direct Applications, B.G. Tuebner, Leipzig, pages 9, 20–22.
- ↑ Maxwell, J.C. (1872). Theory of Heat, third edition, Longmans, Green, and Co., London, page 73.
- ↑ Harper, Douglas. "latent". Online Etymology Dictionary.
- ↑ Lewis, Charlton T. (1890). An Elementary Latin Dictionary. Entry for latens.
- ↑ James Burke (1979). "Credit Where It's Due". The Day the Universe Changed. Episode 6 (in English). 34 minutes in. BBC.
- ↑ J. P. Joule (1884), The Scientific Paper of James Prescott Joule, The Physical Society of London, p. 274,
I am inclined to believe that both of these hypotheses will be found to hold good,—that in some instances, particularly in the case of sensible heat, or such as is indicated by the thermometer, heat will be found to consist in the living force of the particles of the bodies in which it is induced; whilst in others, particularly in the case of latent heat, the phenomena are produced by the separation of particle from particle, so as to cause them to attract one another through a greater space.
, Lecture on Matter, Living Force, and Heat. May 5 and 12, 1847 - ↑ Yaws' Handbook of Properties of the Chemical Elements 2011 Knovel
- ↑ 10.0 10.1 Polynomial curve fits to Table 2.1. R. R. Rogers & M. K. Yau (1989). A Short Course in Cloud Physics (3rd ed.). Pergamon Press. p. 16. ISBN 0-7506-3215-1.
| ||||||||||||||||||||||||||||||||||||