Lanthionine
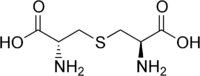 | |
| Identifiers | |
|---|---|
| 922-55-4 | |
| ChEBI | CHEBI:21347 |
| ChemSpider | 88959 |
| |
| Jmol-3D images | Image |
| PubChem | 256406 |
| |
| Properties | |
| C6H12N2O4S | |
| Molar mass | 208.2318 g/mol |
| Melting point | 280 to 283 °C (536 to 541 °F; 553 to 556 K) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). As the monosulfide analog of cystine, lanthionine is composed of two alanine residues that are crosslinked on their β-carbon atoms by a thioether linkage. Despite its name, lanthionine does not contain lanthanum.
Background
In 1941, lanthionine was first isolated from the treatment of wool with sodium carbonate[1] and was first synthesized from cysteine and β-chloroalanine.[2] Lanthionines are found widely in nature and have been isolated from human hair, lactalbumin, and feathers. Lanthionines have also been found in bacterial cell walls and are the components of a group of gene-encoded peptide antibiotics called lantibiotics, which includes nisin (a food preservative), subtilin, epidermin (an anti-staphylococcus and -streptococcus agent), and ancovenin (an enzyme inhibitor).[3][4]
Preparation
A variety of syntheses of lanthionine have been published including sulfur extrusion from cystine,[5] ring opening of serine β-lactone,[4] and hetero-conjugate addition of cysteine to dehydroalanine.[6] The sulfur extrusion method is, however, the only pathway for lanthionine that has been employed in the total synthesis of a lantibiotic.
References
- ↑ Horn, M. J.; Jones, D. B.; Ringel, S. J. (1941) Isolation of a New Sulfur-Containing Amino Acid (Lanthionine) from Sodium Carbonate-Treated Wool. Journal of Biological Chemistry, 138, 141-149.
- ↑ Brown, G. B.; du Vigneaud, V. (1941) The Stereoisomeric Forms of Lanthionine. Journal of Biological Chemistry, 140, 767-771.
- ↑ Paul, M.; van der Donk, W. A. (2005) Chemical and Enzymatic Synthesis of Lanthionines. Mini-Reviews in Organic Chemistry, 2, 23-37.
- ↑ 4.0 4.1 Shao, H.; Wang, S. H. H.; Lee, C.-W.; Ösapay, G.; Goodman, M. (1995) A Facile Synthesis of Orthogonally Protected Stereoisomeric Lanthionines by Regioselective Ring Opening of Serine β-Lactone Derivatives. Journal of Organic Chemistry, 60, 2956-2957.
- ↑ Harpp, D. N.; Gleason, J. G. (1971) Preparation and Mass Spectral Properties of Cystine and Lanthionine Derivatives. Novel Synthesis of L-Lanthionine by Selective Desulfurization. Journal of Organic Chemistry, 36, 73-80.
- ↑ Probert, J. M.; Rennex, D.; Bradley, M. (1996) Lanthionines for Solid Phase Synthesis. Tetrahedron Letters, 37, 1101-1104.