Lansoprazole
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
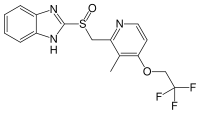
| (RS)-2-([3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl)-1H-benzo[d]imidazole | |
| Clinical data | |
| Trade names | Prevacid |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a695020 |
| Licence data | US FDA:link |
| |
| Oral, IV | |
| Pharmacokinetic data | |
| Bioavailability | 80% or more |
| Protein binding | 97% |
| Metabolism | Hepatic (CYP3A4- and CYP2C19-mediated) |
| Half-life | 1–1.5 hours |
| Excretion | Renal and fecal |
| Identifiers | |
|
103577-45-3 | |
| A02BC03 | |
| PubChem | CID 3883 |
| DrugBank |
DB00448 |
| ChemSpider |
3746 |
| UNII |
0K5C5T2QPG |
| KEGG |
D00355 |
| ChEBI |
CHEBI:6375 |
| ChEMBL |
CHEMBL480 |
| Chemical data | |
| Formula | C16H14F3N3O2S |
| 369.363 g/mol | |
|
SMILES
| |
| |
| | |
Lansoprazole (/lænˈsoʊprəzoʊl/ lan-SOH-prə-zohl; INN) is a proton-pump inhibitor (PPI) which inhibits the stomach's production of gastric acids. It is manufactured by a number of companies worldwide under several brand names. In the United States, it was first approved by the Food and Drug Administration (FDA) in 1995.[1] Prevacid patent protection expired on November 10, 2009.[2][3] Since 2009, lansoprazole has been available over the counter (OTC) in the U.S. in a 15-mg dose marketed by Novartis as Prevacid 24HR.[4][5][6] In Australia, it is marketed by Pfizer as Zoton.
Lansoprazole is a proton-pump inhibitor (PPI) in the same pharmacologic class as omeprazole. Lansoprazole has been marketed for many years and is one of several PPIs available.[7] It is a racemic 1:1 mixture of the enantiomers dexlansoprazole (Dexilant, formerly named Kapidex) and levolansoprazole.[8] Dexlansoprazole is an enantiomerically pure active ingredient of a commercial drug as a result of the 'enantiomeric shift'.
Lansoprazole's plasma elimination half-life (1.5 h) is not proportional to the duration of the drug's effects to the person (i.e. gastric acid suppression).[9] and the effects of the drug last for over 24 hours after it has been used for a day or more.[5] Lansoprazole, 30-mg administered nasogastrically, effectively controls intragastric pH and is an alternative to intravenous pantoprazole in patients who are unable to swallow solid-dose formulations.[10]
Indications
Lansoprazole is indicated for treatment of:
- Ulcers of the stomach and duodenum, and NSAID-induced ulcers
- Helicobacter pylori infection, alongside antibiotics (adjunctive treatment), treatment to kill H. pylori causing ulcers or other problems involves using two other drugs besides lansoprazole known as "triple therapy", and involves taking twice daily for 10 or 14 days lansoprazole 30-mg, amoxicillin 1,000-mg, and clarithromycin 500-mg
- Gastroesophageal reflux disease
- Zollinger-Ellison syndrome[5]
Drug interactions
Lansoprazole interacts with several other drugs, either due to its own nature or as a PPI.[11]
- PPIs reduce absorption of antifungals (itraconazole and ketoconazole) [12] and possibly increase digoxin in plasma
- Increases plasma concentrations of cilostazol (risk of toxicity)
Lansoprazole possibly interacts with, amongst other drugs:
- sucralfate
- ampicillin
- bisacodyl
- clopidogrel
- delavirdine
- fluvoxamine
- iron salts
- voriconazole
- aminophylline and theophylline
- astemizole
Side effects
Side effects of PPIs in general[13] and lansoprazole in particular[14] may include:
- Infrequent: dry mouth, insomnia, drowsiness, blurred vision, rash, pruritus
- Rarely and very rarely: taste disturbance, liver dysfunction, peripheral oedema, hypersensitivity reactions (including bronchospasm, urinary, angioedema, anaphylaxis), photosensitivity, fever, sweating, depression, interstitial nephritis, blood disorders (including leukopenia, leukocytosis, pancytopenia, thrombocytopenia), arthralgia, myalgia, skin reactions[15] including (erythroderma[16] Stevens–Johnson syndrome, toxic epidermal necrolysis, bullous eruption)
- Severe: Gastrointestinal disturbances (such as nausea 1.3%, abdominal pain 2.1%, diarrhea 3.8%)[9]
PPIs may be associated with a greater risk of hip fractures and Clostridium difficile-associated diarrhea.[5]:22
Availability

The lansoprazole molecule is off-patent and so generic drugs are available under many brand names in many countries;[17] there are patents covering some formulations in effect as of 2015.[18]
References
- ↑ Mosby's Drug Consult: Lansoprazole
- ↑ Prevacid drug patents
- ↑ Teva to release Prevacid version when patent expires
- ↑ "Novartis launches Prevacid 24HR over-the-counter for full 24-hour frequent heartburn treatment" (PDF) (Press release). November 12, 2009. Retrieved November 13, 2009.
- ↑ 5.0 5.1 5.2 5.3 "Prevacid 24HR Label" (PDF). May 2010. Retrieved November 15, 2014.
- ↑ "Novartis launches Prevacid 24HR over-the-counter for full 24-hour frequent heartburn treatment" (Press release). November 12, 2009. Retrieved November 13, 2009.
- ↑ http://www.patient.co.uk/showdoc/30002943/
- ↑ "Pharmacy Benefit Update". Retrieved 2 July 2014.
- ↑ 9.0 9.1 "Prevacid Pharmacology, Pharmacokinetics, Studies, Metabolism". RxList.com. 2007. Retrieved April 14, 2007.
- ↑ Effects on 24-Hour Intragastric pHFreston M.D., Ph.D., James; Yi-Lin Chiu, Ph.D., Wei-Jian Pan, Ph.D., Nancy Lukasik, B.S.N., and Jörg Täubel, M.D., A.F.P.M. (2001). "Effects on 24-Hour Intragastric pH: A Comparison of Lansoprazole Administered Nasogastrically in Apple Juice and Pantoprazole Administered Intravenously". American Journal of Gastroenterology 96 (7): 2058–2065. doi:10.1111/j.1572-0241.2001.03939.x. ISSN 0002-9270. OCLC 440925790. PMID 11467632.
- ↑ British National Formulary (Free registration required) Lansoprazole interactions
- ↑ Antimicrob Agents Chemother. 1991 September; 35(9): 1765–1771. Effects of ranitidine and sucralfate on ketoconazole bioavailability. S C Piscitelli, T F Goss, J H Wilton, D T D'Andrea, H Goldstein, and J J Schentag
- ↑ British National Formulary (Free registration required) 1.3.5 Proton pump inhibitors
- ↑ British National Formulary (Free registration required) Lansoprazole
- ↑ K C Singhal & S Z Rahman, Lansoprazole Induced Adverse Effects on the Skin, Indian Medical Gazette, July 2001, Vol. CXXXV. N0. 7: 223-225
- ↑ Sterry W, Assaf C (2007). "Erythroderma". In Bolognia JL. Dermatology. St. Louis: Mosby. p. 154. ISBN 1-4160-2999-0..
- ↑ drugs.com International availability of lansoprazole Page accessed February 3, 2015
- ↑ drugs.com Generic lansoprazole Page accessed February 3, 2015
External links
- Prevacid official website Takeda Pharmaceuticals North America
- Prevacid 24HR official website Novartis Consumer Health
- Prevpac official website Takeda Pharmaceuticals North America
- U.S. National Library of Medicine: Drug Information Portal - Lansoprazole
| ||||||||||||||||||||||||||||||||||||||||||||