L-2-hydroxycarboxylate dehydrogenase (NAD+)
| L-2-hydroxycarboxylate dehydrogenase (NAD+) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.1.1.337 | ||||||||
| CAS number | 81210-65-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
L-2-hydroxycarboxylate dehydrogenase (NAD+) (EC 1.1.1.337, (R)-sulfolactate:NAD+ oxidoreductase, L-sulfolactate dehydrogenase, (R)-sulfolactate dehydrogenase, L-2-hydroxyacid dehydrogenase (NAD+), ComC) is an enzyme with system name (2S)-2-hydroxycarboxylate:NAD+ oxidoreductase.[1][2][3][4] This enzyme catalyses the following chemical reaction
- (2S)-2-hydroxycarboxylate + NAD+
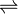 2-oxocarboxylate + NADH + H+
2-oxocarboxylate + NADH + H+
The enzyme from the archaeon Methanocaldococcus jannaschii uses as a substrate multiple (S)-2-hydroxycarboxylates including (2R)-3-sulfolactate, (S)-malate, (S)-lactate, and (S)-2-hydroxyglutarate.
References
- ↑ Graupner, M., Xu, H. and White, R. H. (2000). "Identification of an archaeal 2-hydroxy acid dehydrogenase catalyzing reactions involved in coenzyme biosynthesis in methanoarchaea". J. Bacteriol. 182: 3688–3692. doi:10.1128/JB.182.13.3688-3692.2000. PMC 94539. PMID 10850983.
- ↑ Graupner, M. and White, R. H. (2001). "The first examples of (S)-2-hydroxyacid dehydrogenases catalyzing the transfer of the pro-4S hydrogen of NADH are found in the archaea". Biochim. Biophys. Acta 1548: 169–173. doi:10.1016/S0167-4838(01)00220-5. PMID 11451450.
- ↑ Graham, D. E. and White, R. H. (2002). "Elucidation of methanogenic coenzyme biosyntheses: from spectroscopy to genomics". Nat. Prod. Rep. 19: 133–147. doi:10.1039/b103714p. PMID 12013276.
- ↑ Rein, U., Gueta, R., Denger, K., Ruff, J., Hollemeyer, K. and Cook, A. M. (2005). "Dissimilation of cysteate via 3-sulfolactate sulfo-lyase and a sulfate exporter in Paracoccus pantotrophus NKNCYSA". Microbiology 151 (Pt 3): 737–747. doi:10.1099/mic.0.27548-0. PMID 15758220.
External links
- L-2-hydroxycarboxylate dehydrogenase (NAD ) at the US National Library of Medicine Medical Subject Headings (MeSH)