Kulinkovich reaction
The Kulinkovich reaction describes the organic synthesis of cyclopropanols via reaction of esters with dialkyldialkoxytitanium reagents, generated in situ from Grignard reagents bearing hydrogen in beta-position and titanium(IV) alkoxides such as titanium isopropoxide. This reaction was discovered by Kulinkovich and coworkers in 1989.[1][2][3][4][5][6][7][8] The titanium reagent can be used catalytically.
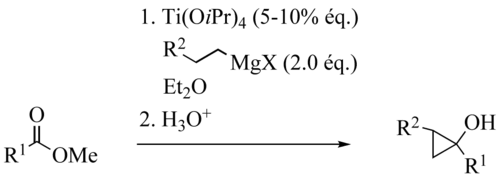
Titanium catalysts are ClTi(OiPr)3 or Ti(OiPr)4, ClTi(OtBu)3 or Ti(OtBu)4, Grignard reagents are EtMgX, PrMgX or BuMgX. Solvents can be Et2O, THF, Toluol. Tolerated Functional Groups: Ethers R–O–R, R–S–R, Imines RN=CHR. Amides, primary and secondary amines, most carbamates are not stable to the reaction conditions, but tert-butyl carbamates (N-Boc derivatives) survive the transformation.
An asymmetric version of this reaction is also known with a TADDOL-based catalyst.[9]
Reaction mechanism
The generally accepted reaction mechanism initially utilizes two successive stages of transmetallation of the committed Grignard reagent, leading to an intermediate dialkyldiisopropyloxytitanium complex. This complex undergoes a dismutation to give an alkane molecule and a titanacyclopropane 1. The insertion of the carbonyl group of the ester in the weakest carbon-titanium bond leads to an oxatitanacyclopentane 2 being rearranged to ketone 3. Lastly, the insertion of the carbonyl group of 3 in the residual carbon-titanium connection forms a cyclopropane ring. In the transition state of this elementary stage, which is the limiting stage of the reaction, an agostic interaction stabilizing between the beta hydrogen and the R2 group and the titanium atom was called upon to explain the diastereoselectivity observed. Complex 4 obtained is a tetraalkyloxytitanium compound able to play a part similar to that of the starting tetraisopropyloxytitanate, which closes the catalytic cycle. At the end of the reaction, the product is mainly in the shape of the magnesium alcoholate 5, giving the cyclopropanol after hydrolysis by the reaction medium.

The reaction mechanism of the Kulinkovich reaction was the subject of thorough calculations published in 2001.[10] Certain points remain nevertheless obscure and the mechanism of this reaction is actually not so simple. Intermediate titanium complexes of the ate type were recently proposed by Kulinkovich.[11]
Ligand exchange with olefins
In 1993, the team of Kulinkovich highlighted the aptitude of the titanacyclopropanes to undergo ligand exchange with olefins.[12] This discovery was important, because it gave access to cyclopropanols more functionalized by making economic use of the Grignard of which normally at least two equivalents should have been engaged to obtain good outputs. Cha and its team introduced the use of cyclic Grignard reagents, particularly adapted for these reactions.[13]
The methodology has been extended to intramolecular reactions[14]
de Meijere variation
With amides instead of esters the reaction product is an aminocyclopropane in the de Meijere variation[15][16]
The intramolecular reaction is also known:[17][18][19][20][21][22][23][24][25][26]
Szymoniak variation
In the Szymoniak variation the substrate is a nitrile and the reaction product a cyclopropane with a primary amine group.[27][28]
The reaction mechanism is akin the Kulinkovich reaction:
References
- ↑ Kulinkovich, O. G.; Sviridov, S. V.; Vasilevskii, D. A.; Pritytskaya, T. S. (1989). Zh. Org. Khim. 25: 2244. Missing or empty
|title=(help) - ↑ Kulinkovich, O. G.; Sviridov, S. V.; Vasilevski, D. A. (1991). "Titanium(IV) Isopropoxide-Catalyzed Formation of 1-Substituted Cyclopropanols in the Reaction of Ethylmagnesium Bromide with Methyl Alkanecarboxylates". Synthesis 1991 (3): 234. doi:10.1055/s-1991-26431.
- ↑ Kulinkovich, O. G.; de Meijere, A. (2000). "1,n-Dicarbanionic Titanium Intermediates from Monocarbanionic Organometallics and Their Application in Organic Synthesis". Chem. Rev. 100 (8): 2789–834. doi:10.1021/cr980046z. PMID 11749306.
- ↑ Sato, F.; Urabe, H.; Okamoto, S. (2000). "Synthesis of organotitanium complexes from alkenes and alkynes and their synthetic applications". Chem. Rev. 100 (8): 2835–86. doi:10.1021/cr990277l. PMID 11749307.
- ↑ Wu, Y.-D.; Yu, Z.-X. (2001). "A theoretical study on the mechanism and diastereoselectivity of the Kulinkovich hydroxycyclopropanation reaction". J. Am. Chem Soc. 123 (24): 5777–86. doi:10.1021/ja010114q. PMID 11403612.
- ↑ Kulinkovich, O. G. (2004). Russ. Chem. Bull 5: 1022–1043. Missing or empty
|title=(help) - ↑ Wolan, A.; Six, Y. (2010). "Synthetic transformations mediated by the combination of titanium(IV) alkoxides and grignard reagents: Selectivity issues and recent applications. Part 1: Reactions of carbonyl derivatives and nitriles". Tetrahedron 66: 15–61. doi:10.1016/j.tet.2009.10.050.
- ↑ Wolan, A.; Six, Y. (2010). "Synthetic transformations mediated by the combination of titanium(IV) alkoxides and Grignard reagents: Selectivity issues and recent applications. Part 2: Reactions of alkenes, allenes and alkynes". Tetrahedron 66 (17): 3097–3133. doi:10.1016/j.tet.2010.01.079.
- ↑ Corey, E. J.; Rao, S. A.; Noe, M. C. (1994). "Catalytic Diastereoselective Synthesis of Cis-1,2-Disubstituted Cyclopropanols from Esters Using a Vicinal Dicarbanion Equivalent". Journal of the American Chemical Society 116 (20): 9345. doi:10.1021/ja00099a068.
- ↑ Wu, Y.–D. and Yu, Z.-X. (2001). "A theoretical study on the mechanism and diastereoselectivity of the Kulinkovich hydroxycyclopropanation reaction". J. Am. Chem. Soc. 123 (24): 5777–86. doi:10.1021/ja010114q. PMID 11403612.
- ↑ Kulinkovich, O. G. and Kananovich, D. G. (2007). "Advanced Procedure for the Preparation ofcis-1,2-Dialkylcyclopropanols – Modified Ate Complex Mechanism for Titanium-Mediated Cyclopropanation of Carboxylic Esters with Grignard Reagents". Eur. J. Org. Chem. 2007 (13): 2121–32. doi:10.1002/ejoc.200601035.
- ↑ Kulinkovich, O. G.; Savchenko, A. I.; Sviridov, S. V. and Vasilevski, D. A. (1993). "Titanium(IV) Isopropoxide-catalysed Reaction of Ethylmagnesium Bromide with Ethyl Acetate in the Presence of Styrene". Mendeleev Commun. 3 (6): 230–31. doi:10.1070/MC1993v003n06ABEH000304.
- ↑ Lee, J.; Kim, H. and Cha, J. K. (1996). "A New Variant of the Kulinkovich Hydroxycyclopropanation. Reductive Coupling of Carboxylic Esters with Terminal Olefins". J. Am. Chem. Soc. 118 (17): 4198–99. doi:10.1021/ja954147f.
- ↑ Kasatkin, A. and Sato, F. (1995). "Diastereoselective synthesis of trans-1,2-disubstituted cyclopropanols from homoallyl or bis-homoallyl esters via tandem intramolecular nucleophilic acyl substitution and intramolecular carbonyl addition reactions mediated by Ti(OPr-i)4 / 2 i-PrMgBr reagent". Tetrahedron Lett. 36 (34): 6079–82. doi:10.1016/0040-4039(95)01208-Y.
- ↑ Chaplinski, V. and de Meijere, A. (1996). "A Versatile New Preparation of Cyclopropylamines from Acid Dialkylamides". Angew. Chem. Int. Ed. 35 (4): 413–14. doi:10.1002/anie.199604131.
- ↑ de Meijere, A.; Winsel, H. and Stecker, B. Organic Syntheses, Vol. 81, p. 14
- ↑ Lee, J.; Cha, J. K. (1997). "Facile Preparation of Cyclopropylamines from Carboxamides". The Journal of Organic Chemistry 62 (6): 1584. doi:10.1021/jo962368d.
- ↑ Chaplinski, V.; Winsel, H.; Kordes, M.; De Meijere, A. (1997). "A New Versatile Reagent for the Synthesis of Cyclopropylamines Including 4-Azaspiro[2.n]alkanes and Bicyclo[n.1.0]alkylamines". Synlett 1997: 111. doi:10.1055/s-1997-17828.
- ↑ Cao, B.; Xiao, D.; Joullié, M. M. (1999). "Synthesis of Bicyclic Cyclopropylamines by Intramolecular Cyclopropanation of N-Allylamino Acid Dimethylamides". Organic Letters 1 (11): 1799. doi:10.1021/ol9910520. PMID 10814442.
- ↑ Lee, H. B.; Sung, M. J.; Blackstock, S. C.; Cha, J. K. (2001). "Radical cation-mediated annulation. Stereoselective construction of bicyclo[5.3.0]decan-3-ones by aerobic oxidation of cyclopropylamines". Journal of the American Chemical Society 123 (45): 11322–11324. doi:10.1021/ja017043f. PMID 11697988.
- ↑ Gensini, M.; Kozhushkov, S. I.; Yufit, D. S.; Howard, J. A. K.; Es-Sayed, M.; Meijere, A. D. (2002). "3-Azabicyclo\3.1.0]hex-1-ylamines by Ti-Mediated Intramolecular Reductive Cyclopropanation of α-(N-Allylamino)-Substituted N,N-Dialkylcarboxamides and Carbonitriles". European Journal of Organic Chemistry 2002 (15): 2499. doi:10.1002/1099-0690(200208)2002:15<2499::AID-EJOC2499>3.0.CO;2-V.
- ↑ Tebben, G. D.; Rauch, K.; Stratmann, C.; Williams, C. M.; De Meijere, A. (2003). "Intramolecular Titanium-Mediated Aminocyclopropanation of Terminal Alkenes: Easy Access to Various Substituted Azabicyclo[n.1.0]alkanes1". Organic Letters 5 (4): 483–485. doi:10.1021/ol027352q. PMID 12583749.
- ↑ Ouhamou, N.; Six, Y. (2003). "Studies on the intramolecular Kulinkovich?de Meijere reaction of disubstituted alkenes bearing carboxylic amide groups". Organic & Biomolecular Chemistry 1 (17): 3007. doi:10.1039/b306719j.
- ↑ Gensini, M.; De Meijere, A. (2004). "Cyclopropane-Annelated Azaoligoheterocycles by Ti-Mediated Intramolecular Reductive Cyclopropanation of Cyclic Amino Acid Amides". Chemistry - A European Journal 10 (3): 785. doi:10.1002/chem.200305068.
- ↑ Larquetoux, L.; Kowalska, J. A.; Six, Y. (2004). "The Formal [3+2+1] Cyclisation of Cyclopropylamines with Carboxylic Anhydrides: A Quick Access to Polysubstituted 2,3,3a,4-Tetrahydro6(5H)-indolone Ring Systems". European Journal of Organic Chemistry 2004 (16): 3517. doi:10.1002/ejoc.200400291.
- ↑ Larquetoux, L.; Ouhamou, N.; Chiaroni, A. L.; Six, Y. (2005). "The Intramolecular Aromatic Electrophilic Substitution of Aminocyclopropanes Prepared by the Kulinkovich-de Meijere Reaction". European Journal of Organic Chemistry 2005 (21): 4654. doi:10.1002/ejoc.200500428.
- ↑ Bertus, P.; Szymoniak, J. (2001). "New and easy route to primary cyclopropylamines from nitriles". Chemical Communications (18): 1792. doi:10.1039/b105293b.
- ↑ Chaplinski, V.; De Meijere, A. (1996). "A Versatile New Preparation of Cyclopropylamines from Acid Dialkylamides". Angewandte Chemie International Edition in English 35 (4): 413. doi:10.1002/anie.199604131.




