Kosmotropic
Co-solvents (in water solvent) are defined as kosmotropic (order-making) if they contribute to the stability and structure of water-water interactions. Kosmotropes cause water molecules to favorably interact, which also (in effect) stabilizes intramolecular interactions in macromolecules such as proteins.[1] Chaotropic agents (disorder-makers) have the opposite effect, disrupting water structure, increasing the solubility of nonpolar solvent particles, and destabilizing solute aggregates.[1]
Ionic Kosmotropes
Ionic kosmotropes tend to be small or have high charge density. Some ionic kosmotropes are CO2−
3, SO2−
4, HPO2−
4, magnesium(2+), lithium(1+), zinc (2+) and aluminium (+3). Large ions or ions with low charge density (such as bromide, iodide, potassium(1+), caesium(1+)) instead act as chaotropes.[2] Kosmotropic anions are more polarizable and hydrate more strongly than kosmotropic cations of the same charge density.[3]
A scale can be established if one refers to the Hofmeister series or looks up the free energy of hydrogen bonding (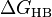 ) of the salts, which quantifies the extent of hydrogen bonding of an ion in water.[4] For example, the kosmotropes CO2−
) of the salts, which quantifies the extent of hydrogen bonding of an ion in water.[4] For example, the kosmotropes CO2−
3 and OH−
have 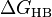 between 0.1 and 0.4 J/mol, whereas the chaotrope SCN−
between 0.1 and 0.4 J/mol, whereas the chaotrope SCN−
has a 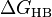 between −1.1 to −0.9.[4]
between −1.1 to −0.9.[4]
Applications
Ammonium sulfate is the traditional kosmotropic salt for the salting out of protein from an aqueous solution. Kosmotropes are used to prevent protein aggregation in pharmaceutical preparation and at various stage of protein extraction and purification. They act by stabilizing native intramolecular protein interactions, thus out-competing the intermolecular interactions that lead to aggregation.
Nonionic Kosmotropes
Nonionic kosmotropes have no net charge but are very soluble and become very hydrated. Carbohydrates such as trehalose and glucose as well as proline and tert-butanol are kosmotropes.
See also
- Chaotropic agent and Guanidinium chloride
- Protein precipitation, on ammonium sulfate "salting out"
External links
- Polson, C; Sarkar, P; Incledon, B; Raguvaran, V; Grant, R (2003). "Optimization of protein precipitation based upon effectiveness of protein removal and ionization effect in liquid chromatography-tandem mass spectrometry". Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 785 (2): 263–275. doi:10.1016/S1570-0232(02)00914-5. PMID 12554139.
References
- ↑ 1.0 1.1 Moelbert S, Normand B, De Los Rios P (2004). "Kosmotropes and chaotropes: modelling preferential exclusion, binding and aggregate stability". BIOPHYSICAL CHEMISTRY 112 (1): 45–57. PMID 15501575.
- ↑ Chaplin, Martin (May 17, 2014). "Kosmotropes and Chaotropes". Water Structure and Science. London South Bank University. Retrieved 2014-09-05.
- ↑ Yang Z (2009). "Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis". JOURNAL OF BIOTECHNOLOGY 144 (1): 12–22. doi:10.1016/j.jbiotec.2009.04.011. PMID 19409939.
- ↑ 4.0 4.1 Marcus Y (2009). "Effect of ions on the structure of water: structure making and breaking". Chemical Reviews 109 (3): 1346–1370. doi:10.1021/cr8003828. PMID 19236019.