Keratin
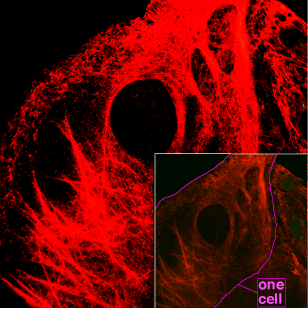
Keratin (/ˈkɛrətɪn/[1][2]) is a family of fibrous structural proteins. Keratin is the key structural material making up the outer layer of human skin. It is the key structural component of hair and nails, and it provides the necessary strength and toughness for masticatory organs, such as the tongue and the hard palate. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong unmineralized tissues found in reptiles, birds, amphibians, and mammals. The only other biological matter known to approximate the toughness of keratinized tissue is chitin.[3][4][5]
Etymology
Keratin derives from Greek κερατίνη from Greek keras (κέρας) (genitive keratos, κέρατος) meaning "horn" originating from the Proto-Indo-European *ḱer- of the same meaning.[6] Its Greek root is composed of "horn like", i.e., kerato (Greek κέρατο)[7] to which the chemical suffix -in is appended.[8]
Examples of uses
Keratin filaments are abundant in keratinocytes in the cornified layer of the epidermis; these are cells which have undergone keratinization. In addition, keratin filaments are present in epithelial cells in general. For example, mouse thymic epithelial cells (TECs) are known to react with antibodies for keratin 5, keratin 8, and keratin 14. These antibodies are used as fluorescent markers to distinguish subsets of TECs in genetic studies of the thymus.
- the α-keratins in the hair (including wool), horns, nails, claws and hooves of mammals
- the harder β-keratins found in nails and in the scales and claws of reptiles, their shells (Testudines, such as tortoise, turtle, terrapin), and in the feathers, beaks, claws of birds and quills of porcupines.[9] (These keratins are formed primarily in beta sheets. However, beta sheets are also found in α-keratins.)[10]
The baleen plates of filter-feeding whales are made of keratin.
Keratins (also described as cytokeratins) are polymers of type III and type IV intermediate filaments, which have only been found in the genomes of chordates (vertebrates, Amphioxus, urochordates). Nematodes and many other non-chordate animals seem to only have type VI intermediate filaments, lamins, which have a long rod domain (vs. a short rod domain for the keratins).
Structural details

The first sequences of keratins were determined by Hanukoglu and Fuchs.[11] [12] These sequences revealed that there are two distinct but homologous keratin families which were named as Type I keratin and Type II keratins.[12] By analysis of the primary structures of these keratins and other intermediate filament proteins, Hanukoglu and Fuchs suggested a model that keratins and intermediate filaments proteins contain a central ~310 residue domain with four segments in α-helical conformation that are separated by three short linker segments predicted to be in beta-turn conformation.[12] This model has been confirmed by the determination of the crystal structure of a helical domain of keratins.[13]
Fibrous keratin molecules supercoil to form a very stable, left-handed superhelical motif to multimerise, forming filaments consisting of multiple copies of the keratin monomer.[14]
The major force that keeps the coiled-coil structure is hydrophobic interactions between apolar residues along the keratins helical segments.[15]
Limited interior space is the reason why the triple helix of the (unrelated) structural protein collagen, found in skin, cartilage and bone, likewise has a high percentage of glycine. The connective tissue protein elastin also has a high percentage of both glycine and alanine. Silk fibroin, considered a β-keratin, can have these two as 75–80% of the total, with 10–15% serine, with the rest having bulky side groups. The chains are antiparallel, with an alternating C → N orientation.[16] A preponderance of amino acids with small, nonreactive side groups is characteristic for structural proteins, for which H-bonded close packing is more important than chemical specificity.
Disulfide bridges
In addition to intra- and intermolecular hydrogen bonds, keratins have large amounts of the sulfur-containing amino acid cysteine, required for the disulfide bridges that confer additional strength and rigidity by permanent, thermally stable crosslinking[17]—a role sulfur bridges also play in vulcanized rubber. Human hair is approximately 14% cysteine. The pungent smells of burning hair and rubber are due to the sulfur compounds formed. Extensive disulfide bonding contributes to the insolubility of keratins, except in dissociating or reducing agents.
The more flexible and elastic keratins of hair have fewer interchain disulfide bridges than the keratins in mammalian fingernails, hooves and claws (homologous structures), which are harder and more like their analogs in other vertebrate classes. Hair and other α-keratins consist of α-helically coiled single protein strands (with regular intra-chain H-bonding), which are then further twisted into superhelical ropes that may be further coiled. The β-keratins of reptiles and birds have β-pleated sheets twisted together, then stabilized and hardened by disulfide bridges.
Filament formation
It was theorized that keratins are combined into 'hard' and 'soft,' or 'cytokeratins' and 'other keratins'. That model is now understood to be correct. A new nuclear addition in 2006 to describe keratins takes this into account.[18]
Keratin filaments are intermediate filaments. Like all intermediate filaments, keratin proteins form filamentous polymers in a series of assembly steps beginning with dimerization; dimers assemble into tetramers and octamers and eventually, if the current hypothesis holds, into unit-length-filaments (ULF) capable of annealing end-to-end into long filaments.
Pairing
| A (neutral-basic) | B (acidic) | Occurrence |
|---|---|---|
| keratin 1, keratin 2 | keratin 9, keratin 10 | stratum corneum, keratinocytes |
| keratin 3 | keratin 12 | cornea |
| keratin 4 | keratin 13 | stratified epithelium |
| keratin 5 | keratin 14, keratin 15 | stratified epithelium |
| keratin 6 | keratin 16, keratin 17 | squamous epithelium |
| keratin 7 | keratin 19 | ductal epithelia |
| keratin 8 | keratin 18, keratin 20 | simple epithelium |
The entries KRT23, KRT24, KRT25, KRT26, KRT27, KRT28, KRT31, KRT32, KRT33A, KRT33B, KRT34, KRT35, KRT36, KRT37, KRT38, KRT39, KRT40, KRT71, KRT72, KRT73, KRT74, KRT75, KRT76, KRT77, KRT78, KRT79, KRT8, KRT80, KRT81, KRT82, KRT83, KRT84, KRT85 and KRT86 have been used to describe keratins past 20.[18]
Cornification
Cornification is the process of forming an epidermal barrier in stratified squamous epithelial tissue. At the cellular level, cornification is characterised by:
- production of keratin
- production of small proline-rich (SPRR) proteins and transglutaminase which eventually form a cornified cell envelope beneath the plasma membrane
- terminal differentiation
- loss of nuclei and organelles, in the final stages of cornification
Metabolism ceases, and the cells are almost completely filled by keratin. During the process of epithelial differentiation, cells become cornified as keratin protein is incorporated into longer keratin intermediate filaments. Eventually the nucleus and cytoplasmic organelles disappear, metabolism ceases and cells undergo a programmed death as they become fully keratinized. In many other cell types, such as cells of the dermis, keratin filaments and other intermediate filaments function as part of the cytoskeleton to mechanically stabilize the cell against physical stress. It does this through connections to desmosomes, cell-cell junctional plaques, and hemidesmosomes, cell-basement membrane adhesive structures.
Cells in the epidermis contain a structural matrix of keratin, which makes this outermost layer of the skin almost waterproof, and along with collagen and elastin, gives skin its strength. Rubbing and pressure cause thickening of the outer, cornified layer of the epidermis and form protective calluses — useful for athletes and on the fingertips of musicians who play stringed instruments. Keratinized epidermal cells are constantly shed and replaced.
These hard, integumentary structures are formed by intercellular cementing of fibers formed from the dead, cornified cells generated by specialized beds deep within the skin. Hair grows continuously and feathers moult and regenerate. The constituent proteins may be phylogenetically homologous but differ somewhat in chemical structure and supermolecular organization. The evolutionary relationships are complex and only partially known. Multiple genes have been identified for the β-keratins in feathers, and this is probably characteristic of all keratins.
Silk
The silk fibroins produced by insects and spiders are often classified as keratins, though it is unclear whether they are phylogenetically related to vertebrate keratins.
Silk found in insect pupae, and in spider webs and egg casings, also has twisted β-pleated sheets incorporated into fibers wound into larger supermolecular aggregates. The structure of the spinnerets on spiders’ tails, and the contributions of their interior glands, provide remarkable control of fast extrusion. Spider silk is typically about 1 to 2 micrometres (µm) thick, compared with about 60 µm for human hair, and more for some mammals. The biologically and commercially useful properties of silk fibers depend on the organization of multiple adjacent protein chains into hard, crystalline regions of varying size, alternating with flexible, amorphous regions where the chains are randomly coiled.[19] A somewhat analogous situation occurs with synthetic polymers such as nylon, developed as a silk substitute. Silk from the hornet cocoon contains doublets about 10 µm across, with cores and coating, and may be arranged in up to 10 layers; also in plaques of variable shape. Adult hornets also use silk as a glue, as do spiders.
Clinical significance
Some infectious fungi, such as those that cause athlete's foot and ringworm (i.e. the dermatophytes), or Batrachochytrium dendrobatidis (Chytrid fungus), feed on keratin.
Diseases caused by mutations in the keratin genes include
- Epidermolysis bullosa simplex
- Ichthyosis bullosa of Siemens
- Epidermolytic hyperkeratosis
- Steatocystoma multiplex
- Keratosis pharyngis
- Rhabdoid cell formation in Large cell lung carcinoma with rhabdoid phenotype[20][21]
Furthermore, keratin expression is helpful in determining epithelial origin in anaplastic cancers. Tumors that express keratin include carcinomas, thymomas, sarcomas and trophoblastic neoplasms. Furthermore, the precise expression pattern of keratin subtypes allows prediction of the origin of the primary tumor when assessing metastases. For example, hepatocellular carcinomas typically expresse K8 and K18, and cholangiocarcinomas express K7, K8 and K18, while metastases of colorectal carcinomas express K20, but not K7.[22]
Cosmetic uses
Hydrolysed keratin has become a common cosmetic ingredient. Studies have shown topical application of hydrolysed keratin gives significant increases in skin elasticity and hydration.[23] Due to its moisturising properties, hydrolysed keratin has also been incorporated into shampoo and conditioner.
Larger keratin structures such as those formed by cornification cannot penetrate the skin so cannot be used as moisturisers. However there are other uses, from hair loss concealing products using fine hair fibres[24] to hair thickening accessories like hair extensions.
See also
- List of cutaneous conditions caused by mutations in keratins
- List of keratins expressed in the human integumentary system
References
- ↑ OED 2nd edition, 1989 as /ˈkɛrətɪn/.
- ↑ Entry "keratin" in Merriam-Webster Online Dictionary.
- ↑ "Keratin". Webster's Online Dictionary.
- ↑ Vincenta, Julian F.V.; Wegst, Ulrike G.K. (1 July 2004). "Design and mechanical properties of insect cuticle" (PDF). Arthropod Structure & Development 33 (3): 187–199. doi:10.1016/j.asd.2004.05.006.
- ↑ Tombolato L, Novitskaya EE, Chen PY, Sheppard FA, McKittrick J (February 2010). "Microstructure, elastic properties and deformation mechanisms of horn keratin" (PDF). Acta Biomater 6 (2): 319–30. doi:10.1016/j.actbio.2009.06.033. PMID 19577667.
- ↑ "Keratin". Online Etymology Dictionary.
"Horn". Online Etymology Dictionary. - ↑ "kerato-". Online Etymology Dictionary.
"Horn". Online Etymology Dictionary. - ↑ "-in/-ine chemical suffix". Online Etymology Dictionary.
- ↑ Hickman, Cleveland Pendleton; Roberts, Larry S.; Larson, Allan L. (2003). Integrated principles of zoology. Dubuque, IA: McGraw-Hill. p. 538. ISBN 0-07-243940-8.
- ↑ Kreplak L, Doucet J, Dumas P, Briki F (2004). "New aspects of the alpha-helix to beta-sheet transition in stretched hard alpha-keratin fibers". Biophys J 87 (1): 640–7. doi:10.1529/biophysj.103.036749. PMC 1304386. PMID 15240497.
- ↑ Hanukoglu, I.; Fuchs, E. (Nov 1982). "The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins.". Cell 31 (1): 243–52. doi:10.1016/0092-8674(82)90424-X. PMID 6186381.
- ↑ 12.0 12.1 12.2 Hanukoglu, I.; Fuchs, E. (Jul 1983). "The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins.". Cell 33 (3): 915–24. doi:10.1016/0092-8674(83)90034-X. PMID 6191871.
- ↑ Lee, CH.; Kim, MS.; Chung, BM.; Leahy, DJ.; Coulombe, PA. (Jul 2012). "Structural basis for heteromeric assembly and perinuclear organization of keratin filaments.". Nat Struct Mol Biol 19 (7): 707–15. doi:10.1038/nsmb.2330. PMID 22705788.
- ↑ Voet, Donald; Voet, Judith; Pratt, Charlotte. "Proteins: Three-Dimensional Structure" (PDF). Fundamentals of Biochemistry. p. 158. Retrieved 2010-10-01.
Fibrous proteins are characterized by a single type of secondary structure: a keratin is a left-handed coil of two a helices
- ↑ Hanukoglu, I.; Ezra, L. (Jan 2014). "Proteopedia: Coiled-coil structure of keratins.". Biochem Mol Biol Educ 42 (1): 93–94. doi:10.1002/bmb.20746. PMID 24265184.
- ↑ "Secondary Protein". Elmhurst.edu. Retrieved 2010-09-23.
- ↑ "What is Keratin?". WiseGEEK. Retrieved 11 May 2014.
- ↑ 18.0 18.1 Schweizer J, Bowden PE, Coulombe PA et al. (July 2006). "New consensus nomenclature for mammalian keratins". J. Cell Biol. 174 (2): 169–74. doi:10.1083/jcb.200603161. PMC 2064177. PMID 16831889.
- ↑ Australia. "Spiders - Silk structure". Amonline.net.au. Retrieved 2010-09-23.
- ↑ Shiratsuchi H, Saito T, Sakamoto A et al. (February 2002). "Mutation analysis of human cytokeratin 8 gene in malignant rhabdoid tumor: a possible association with intracytoplasmic inclusion body formation". Mod. Pathol. 15 (2): 146–53. doi:10.1038/modpathol.3880506. PMID 11850543.
- ↑ Itakura E, Tamiya S, Morita K et al. (September 2001). "Subcellular distribution of cytokeratin and vimentin in malignant rhabdoid tumor: three-dimensional imaging with confocal laser scanning microscopy and double immunofluorescence". Mod. Pathol. 14 (9): 854–61. doi:10.1038/modpathol.3880401. PMID 11557780.
- ↑ Omary MB, Ku NO, Strnad P, Hanada S (July 2009). "Toward unraveling the complexity of simple epithelial keratins in human disease". J. Clin. Invest. 119 (7): 1794–805. doi:10.1172/JCI37762. PMC 2701867. PMID 19587454.
- ↑ Barba C, Méndez S, Roddick-Lanzilotta A, Kelly R, Parra JL, Coderch L. (May 2008). "Cosmetic effectiveness of topically applied hydrolysed keratin peptides and lipids derived from wool.". Skin Res. Technol. 14 (2): 243–248. doi:10.1111/j.1600-0846.2007.00280. PMID 18412569.
- ↑ Pangaea Laboratories. "Hair Fibres". Retrieved 2014-02-18.
External links
| Wikisource has the text of the 1920 Encyclopedia Americana article Keratin. |
- Composition and β-sheet structure of silk
- Hair-Science.com's entry on the microscopic elements of hair
- Proteopedia page on keratins
| ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
