Kaminsky catalyst
A Kaminsky catalyst is a catalytic system for alkene polymerization discovered in 1980. Kaminsky catalysts are based on metallocenes of group 4 transition metals with methylaluminoxane (MAO). These catalysts are a type of Ziegler-Natta catalyst, but they are homogeneous and show extremely high activity for polymerization of olefins such as ethylene and propylene. In addition, the use of chiral metallocenes that have bridged cyclopentadienyl rings has made possible highly stereoselective (or stereoregular) polymerization of α-olefins. For example, by using metallocene 1 for polymerization of propylene, atactic polypropylene is obtained, while C2 symmetric metallocene 2 and Cs symmetric metallocene 3 catalytic systems produce isotactic polymer and syndiotactic macromolecule, respectively.
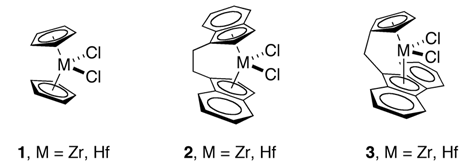
Kaminsky catalysts are very attractive from the industrial, organometallic, and polymer science points of view, and they are studied to improve their activity and to achieve high tacticity and high molecular weight of the produced polymers.
Synthesis
MAO undergoes a rapid ligand exchange with a metallocene dichloride. This produces a metallocene methyl and dimethyl aluminum compound. After this ligand exchange either a chlorine or methyl is abstracted from the metallocene by one of the aluminum centers in MAO. This process of abstraction produces a metallocene cation as well as a MAO anion. The resultant alkylated metallocene is the active center.
Metallocene Catalysts
Metallocene catalysts are soluble in hydrocarbons. These complexes can be easily modified and their structure includes only one active site which allows for a very stereoselective approach to polymerization. This class of catalysts allows for someone to correctly predict the properties of a polyolefin produced through polymerization simply by knowing the properties of the catalyst used to produce said polyolefin, such as molecular weight and distribution of ligands. The Kaminsky catalyst is 10 to 100 times more catalytically active than its close relative the Ziegler-Natta catalysts.[1] These compounds are known as sandwich comounds due to the fact that they consist of a pi-bonded metal situated between two aromatic ring systems. Metallocene complexes along with conventional aluminum alkyl catalysts can polymerize ethylene, although rather slowly. In the future the possibility of producing more structures as well as a new string of polymers becomes more and more real. The catalysts that are most likely to be used for these future developments are most likely to have metallocenes as their main component.
Methylaluminoxane (MAO)
Methylaluminoxane (MAO) was discovered at the University of Hamburg in 1977. This substance is made by partial hydrolysis of trimethylaluminum and consists mainly of units of [Al4O3Me6]. The molecular weight of (MAO) can vary between 1200-1600 (g/mol) depending on how many units of [Al4O3Me6] are present. (MAO) was found to drastically enhance the activity of polymerization, nearly 10,000 times that of previously discovered compounds. This compound, Methylaluminoxane, plays a major role in catalysis with metallocenes. The structure of (MAO) consists of aluminum and oxygen atoms arranged alternately, as well as free valence electrons that are saturated by methyl substitutes.
References
- ↑ Highly Active Metallocene Catalysts For Olefin Polymerization Walter Kaminsky J. Chem. Soc. 1998
Additional Reading
- Multinuclear Group 4 Catalysis: Olefin Polymerization Pathways Modified by Strong Metal-Metal Cooperative Effects McInnis, J.; Delferro, M.; Marks, T. Acc. Chem. Res. 47 (8) 2545-2557 doi:10.1021/AR5001633
- Binding of polar monomers in the complexes with organometallic ethylene polymerization catalysts-Natural orbitals for chemical balence and energy decomposition analysis Srebro, M.; Mitoraj, M.; Michalak, A. Canadian Journal of Chemistry 87 (7) 1039-1054 doi:10.1139/V09-072
- Theoretical studies on the role of bridging group of CGC type ligands for the Ziegler-Natta catalysis Sakai, S.; Kojima, Y. Journal of Organometallic Chemistry 964 (20) 3276-3280