John Alexander Reina Newlands
| John Alexander Reina Newlands | |
|---|---|
 | |
| Born |
26 November 1837 West Square, Southwark, London, England |
| Died |
29 July 1898 (aged 60) Lower Clapton, London, England |
| Nationality | British |
| Fields | Analytical chemistry |
| Alma mater | Royal College of Chemistry |
| Known for | Periodic table, law of octaves |
| Notable awards | Davy Medal (1887) |
John Alexander Reina Newlands (26 November 1837 – 29 July 1898) was an English chemist who worked on the development of the periodic table.
Life
Newlands was born in London, England, and was the son of a Scottish Presbyterian minister and his Italian wife.[1] He was homeschooled by his father and went on to study at the Royal College of Chemistry. He was interested in social reform and in 1860 served as a volunteer with Giuseppe Garibaldi in his campaign to unify Italy. Returning to London, Newlands set up in practice as an analytical chemist in 1864, and in 1868 he became chief chemist in James Duncan's London sugar refinery, where he introduced a number of improvements in processing. Later he left the refinery and again set up as an analyst with his brother, Benjamin.
Newlands was the first person to devise a periodic table of elements arranged in order of their relative atomic weights.[2] Continuing Johann Wolfgang Döbereiner’s work with triads and Jean-Baptiste Dumas' families of similar elements, he published in 1865 his 'Law of octaves', which stated that "any given element will exhibit analogous behaviour to the eighth element following it in the table." Newlands arranged all of the known elements into seven groups, which he likened to the octaves of music.[3][4] In Newlands' table, the elements were ordered by the atomic weights that were known at the time and were numbered sequentially to show their order. Periods were shown going down the table, with groups going across – the opposite from the modern form of the periodic table.
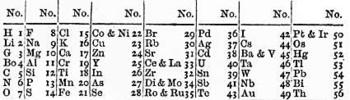
The incompleteness of the table alluded to the possible existence of additional, undiscovered elements, such as the element germanium, which was predicted by Newlands.
At the time, his 'Law of octaves' was ridiculed by his contemporaries. The Society of Chemists did not accept his work for publication.[5]
After Dmitri Mendeleev and Lothar Meyer received the Davy Medal from the Royal Society for their later 'discovery' of the Periodic table, Newlands fought for recognition of his earlier work and eventually received the Davy medal in 1887.
John Newlands died on 29 July 1898 at his home in Lower Clapton, London, and was buried at West Norwood Cemetery. His business was continued after his death by his younger brother, Benjamin.
Newland's law of octaves
In the 1860s, John Newlands arranged the then known elements in the order of increasing masses. He started with the element having the least atomic mass (hydrogen) and ended at thorium which was the 56th element. He found that every eighth element had properties similar to that of the first. He compared this to the octaves in music. Therefore, he called it the 'Law of Octaves'. However there were many drawbacks to this system.
See also
References
- ↑ 'Newlands, John Alexander Reina (1837–1898)' by Michael A. Sutton, Oxford Dictionary of National Biography, Oxford University Press, 2004. Accessed 5 February 2011.
- ↑ Like many of his contemporaries, Newlands first used the terms equivalent weight and atomic weight without any distinction in meaning and in his first paper in 1863.He used the values accepted by his predecessors. It is now referred to as relative atomic mass.
- ↑ Newlands, John A. R. (1864-08-20). "On Relations Among the Equivalents". Chemical News 10: 94–95.
- ↑ Newlands, John A. R. (1865-08-18). "On the Law of Octaves". Chemical News 12: 83.
- ↑ Bryson, Bill (2004). A Short History of Nearly Everything. London: Black Swan. pp. 141–142. ISBN 978-0-552-15174-0.
Further reading
- Scerri, Eric R. (2007). The periodic table: Its story and its significance. Oxford: Oxford University Press. ISBN 0-19-530573-6.