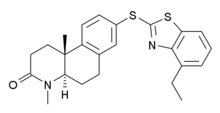
Izonsteride
Izonsteride|
|
| Systematic (IUPAC) name |
|---|
|
(4aR,10bR)-8-[(4-ethyl-1,3-benzothiazol-2-yl)sulfanyl]-4,10b-dimethyl-1,4,4a,5,6,10b-hexahydrobenzo[f]quinolin-3(2H)-one |
| Clinical data |
|---|
|
|
|---|
|
Oral |
|---|
| Identifiers |
|---|
|
176975-26-1 |
|---|
|
None |
|---|
| PubChem |
CID 172988 |
|---|
| ChemSpider |
151061 |
|---|
| UNII |
A5E8C36F34  Y Y |
|---|
| Chemical data |
|---|
| Formula |
C24H26N2OS2 |
|---|
|
422.61 g/mol |
|---|
SMILES
- O=C4N(C)[C@@H]5CCc3cc(Sc1nc2c(cccc2s1)CC)ccc3[C@@]5(C)CC4
|
Izonsteride (LY-320,236) is a selective inhibitor of the 5α-reductase, with dual effects on both the type I and type II isoforms of the enzyme.[1] It was under development by Eli Lilly and Company and Fujisawa for the treatment of benign prostatic hyperplasia but was never marketed.[2] Izonsteride may also be useful in the treatment of androgenic alopecia.[1]
Synthesis
The scheme used to produce a somewhat more complex 5-a-reductase inhibitor relies on a chiral auxiliary to yield the final product as a single enantiomer. The first step starts with the reaction of bromotetralone with R-α-phenethylamine to afford the enamine. Reaction with methyl iodide adds the methyl group at what will be a steroid-like AB ring junction.
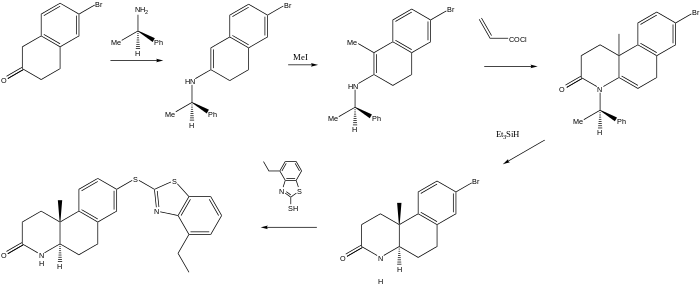
Preparation of Izonsteride
This product is then treated with acryloyl chloride. The initial step in this case probably involves the acylation of nitrogen on the enamine; conjugate addition then completes the formation of the lactam ring. Treatment of that product with triethyl silane then reduces the ring unsaturation and cleaves the benzylic nitrogen bond on the auxiliary to yield as the optically pure trans isomer. Displacement of bromine with the mercapto benzthiazole completes the synthesis of izonsteride.[3]
See also
References
- ↑ 1.0 1.1 Chang, Chawnshang (2002). Androgens and androgen receptor : mechanisms, functions, and clinical application. Boston: Kluwer Academic Publishers. ISBN 1-4020-7188-4.
- ↑ "Present and future pharmacotherapy for benign prostatic hyperplasia".
- ↑ Audia, J. R.; McQuaid, L. A.; Neubauer, B. L.; Rocco, V. P.; 1997, U.S. Patent 5,662,962.
|
|---|
| | 5α-reductase inhibitors | |
|---|
| | Alpha blockers (α1) | |
|---|
| | Herbals | |
|---|
| | Other | |
|---|
| |
|---|
| | Description |
- Anatomy
- Physiology
- Development
- sex determination and differentiation
|
|---|
| | Disease |
- Infections
- Congenital
- Neoplasms and cancer
- male
- female
- gonadal
- germ cell
- Other
- Symptoms and signs
|
|---|
| | Treatment |
- Procedures
- Drugs
- benign prostatic hypertrophy
- erectile dysfunction and premature ejaculation
- sexual dysfunction
- infection
- hormones
- androgens
- estrogens
- progestogens
- GnRH
- prolactin
- Assisted reproduction
- Birth control
|
|---|
|
|
Other dermatological preparations ( D11) |
|---|
| | Medicated shampoos | |
|---|
| | Other dermatologicals | |
|---|
| |
|---|
| | Description |
- Anatomy
- Physiology
- Development
|
|---|
| | Disease |
- Infections
- Vesiculobullous
- Dermatitis and eczema
- Papulosquamous
- Urticaria and erythema
- Radiation-related
- Pigmentation
- Mucinoses
- Keratosis, ulcer, atrophy, and necrobiosis
- Vasculitis
- Fat
- Neutrophilic and eosinophilic
- Congenital
- Neoplasms and cancer
- nevi and melanomas
- epidermis
- dermis
- Symptoms and signs
- Terminology
|
|---|
| | Treatment |
- Procedures
- Drugs
- antibiotics
- disinfectants
- emollients and protectives
- itch
- psoriasis
- other
- Wound and ulcer
|
|---|
|
|---|
| | Description |
- Anatomy
- Physiology
- Development
|
|---|
| | Disease |
- Congenital
- Neoplasms and cancer
- Other
- Symptoms and signs
|
|---|
| | Treatment | |
|---|
|
|
|
|---|
| | Receptor | |
|---|
| | Enzyme | |
|---|
| | Others |
- Indirect: Antigonadotropins (e.g., estrogens, progestogens, prolactin)
- GnRH agonists (e,g, GnRH, leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Gonadotropins (e.g., FSH, hCG, LH)
- Kisspeptin
- Plasma proteins (ABP, albumin, SHBG)
|
|---|
| See also: Estrogenics • Glucocorticoidics • Mineralocorticoidics • Progestogenics |
|