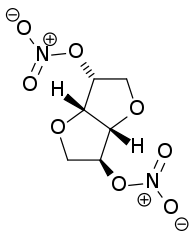
Isosorbide dinitrate
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
| 1,4:3,6-dianhydro-2,5-di-O-nitro-D-glucitol | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| |
| |
| Oral | |
| Pharmacokinetic data | |
| Bioavailability | 10–90%, average 25% |
| Metabolism | Hepatic |
| Half-life | 1 hour |
| Excretion | Renal |
| Identifiers | |
|
87-33-2 | |
| C01DA08 C05AE02 | |
| PubChem | CID 6883 |
| DrugBank |
DB00883 |
| ChemSpider |
6619 |
| UNII |
IA7306519N |
| KEGG |
D00516 |
| ChEBI |
CHEBI:6061 |
| ChEMBL |
CHEMBL2171310 |
| Synonyms | (3R,3aS,6S,6aS)-hexahydrofuro[3,2-b]furan-3,6-diyl dinitrate |
| Chemical data | |
| Formula | C6H8N2O8 |
| 236.136 g/mol | |
|
SMILES
| |
| |
| | |
Isosorbide dinitrate, ISDN, is a nitrate used pharmacologically as a vasodilator, e.g. in angina pectoris, but also for anal fissure, a condition known to involve decreased blood supply leading to poor healing. It is also used as a direct vasodilator to treat congestive heart failure.
It is used for heart-related chest pain, in addition to other medications for congestive heart failure, and for esophageal spasms.[1]
Isosorbide dinitrate is sold in the USA under the brand names Dilatrate-SR by Schwarz and Isordil by Valeant, according to FDA Orange Book. In the United Kingdom, Argentina, and Hong Kong, a trade name of it is Isoket. It is also a component of BiDil. It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[2]
Medical uses
It is used for angina, in addition to other medications for congestive heart failure, and for esophageal spasms.[1]
Advantages
Long-acting nitrates can be more useful as they are generally more effective and stable in the short term.
Disadvantages
After long-term use for treating chronic conditions, tolerance may develop in patients, reducing its effectiveness. The mechanisms of nitrate tolerance have been thoroughly investigated in the last 30 years and several hypotheses have been proposed. These include:
- Impaired biotransformation of ISDN to its active principle NO (or a NO-related species)
- Neurohormonal activation, causing sympathetic activation and release of vasoconstrictors such as endothelin and angiotensin II which counteract the vasodilation induced by ISDN
- Plasma volume expansion
- The oxidative stress hypothesis (proposed by Munzel et al. in 1995)
The last hypothesis might represent a unifying hypothesis, and an ISDN-induced inappropriate production of oxygen free radicals might induce a number of abnormalities which include the ones described above. Furthermore, nitrate tolerance is shown to be associated with vascular abnormalities which have the potential to worsen patients prognosis: [3] these include endothelial and autonomic dysfunction. [4] In the short run, ISDN can cause severe headaches, necessitating analgesic (very rarely up to morphine) administration for relief of pain, as well as severe hypotension, and, in certain cases, bradycardia. This makes some physicians nervous and should prompt caution when starting nitrate administration.
Synthesis

References
- ↑ 1.0 1.1 "Isosorbide Dinitrate/Mononitrate". The American Society of Health-System Pharmacists. Retrieved Apr 21, 2014.
- ↑ "WHO Model List of EssentialMedicines". World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ (Nakamura et al.)
- ↑ (Gori et al.).
- ↑ Goldberg, L. (1948). "Pharmacological Properties of Sorbide Dinitrate". Acta Physiologica Scandinavica 15 (2): 173–87. doi:10.1111/j.1748-1716.1948.tb00494.x. PMID 18863140.
- ↑ Pharmacol Exp Ther October 1939 67:187-190.
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||