Isorhapontigenin
 | |
| Names | |
|---|---|
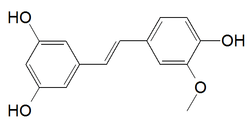
| IUPAC name
5-[(E)-2-(4-hydroxy-3-methoxyphenyl)ethenyl]benzene-1,3-diol | |
| Other names
3,4',5-trihydroxy-3'-methoxystilbene ISO | |
| Identifiers | |
| 32507-66-7 | |
| ChemSpider | 4477172 |
| |
| Jmol-3D images | Image |
| PubChem | 5318650 |
| |
| Properties | |
| C15H14O4 | |
| Molar mass | 258.27 g/mol |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Isorhapontigenin is a tetrahydroxylated stilbenoid with a methoxy group. It is an isomer of rhapontigenin and an analog of resveratrol.[1] It is found in the Chinese herb Gnetum cleistostachyum,[2] in Gnetum parvifolium[3] and in the seeds of the palm Aiphanes aculeata.[4]
An isorhapontigenin tetramer, gnetuhainin R, can be isolated from the lianas of Gnetum hainanense.[5]
Isorhapontin, the isorhapontigenin glucoside, can be found in spruce species such as the Norway spruce (Picea abies), the sitka spruce (Picea sitchensis) and the white spruce (Picea glauca).[6]
References
- ↑ Li, H.; Wang, A.; Huang, Y.; Liu, D.; Wei, C.; Williams, G.; Zhang, C.; Liu, G.; Liu, Y.; Hao, D.; Hui, R. T.; Lin, M.; Liang, C. C. (2005). "Isorhapontigenin, a new resveratrol analog, attenuates cardiac hypertrophy via blocking signaling transduction pathways". Free Radical Biology and Medicine 38 (2): 243–257. doi:10.1016/j.freeradbiomed.2004.10.020. PMID 15607907.
- ↑ Fang, Y.; Yu, Y.; Hou, Q.; Zheng, X.; Zhang, M.; Zhang, D.; Li, J.; Wu, X. -R.; Huang, C. (2012). "The Chinese Herb Isolate Isorhapontigenin Induces Apoptosis in Human Cancer Cells by Down-regulating Overexpression of Antiapoptotic Protein XIAP". Journal of Biological Chemistry 287 (42): 35234–35243. doi:10.1074/jbc.M112.389494. PMC 3471739. PMID 22896709.
- ↑ Piao, Z. S.; Feng, Y. B.; Wang, L.; Zhang, X. Q.; Lin, M. (2010). "Synthesis and HIV-1 inhibitory activity of natural products isolated from Gnetum parvifolium and their analogues". Yao xue xue bao = Acta pharmaceutica Sinica 45 (12): 1509–1515. PMID 21351490. (article in Chinese)
- ↑ Lee, D.; Cuendet, M.; Vigo, J. S.; Graham, J. G.; Cabieses, F.; Fong, H. H.; Pezzuto, J. M.; Kinghorn, A. D. (2001). "A novel cyclooxygenase-inhibitory stilbenolignan from the seeds of Aiphanes aculeata". Organic letters 3 (14): 2169–2171. doi:10.1021/ol015985j. PMID 11440571.
- ↑ Huang, K. S.; Zhou, S.; Lin, M.; Wang, Y. H. (2002). "An Isorhapontigenin Tetramer and a Novel Stilbene Dimer fromGnetum hainanense". Planta Medica 68 (10): 916–920. doi:10.1055/s-2002-34951. PMID 12391556.
- ↑ Hammerbacher, A.; Ralph, S. G.; Bohlmann, J.; Fenning, T. M.; Gershenzon, J.; Schmidt, A. (2011). "Biosynthesis of the Major Tetrahydroxystilbenes in Spruce, Astringin and Isorhapontin, Proceeds via Resveratrol and is Enhanced by Fungal Infection". Plant Physiology 157 (2): 876–890. doi:10.1104/pp.111.181420. PMC 3192583. PMID 21865488.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||