Isoelectronicity
Two or more molecular entities (atoms, molecules, or ions) are described as being isoelectronic with each other if they have the same number of electrons[1] or a similar electron configuration[2] and the same structure (number and connectivity of atoms), regardless of the nature of the elements involved.
The term valence isoelectronic is used when these molecular entities have the same number of valence electrons or a similar electron configuration, but may have a different number of atoms or a different bonding.[3]
The statement "These compounds or molecules are isoelectronic" is not just an implementation of the above definition. It has significance by the fact that calculations on molecules and electron density, and therefore capability of reaction, have been performed on many common substances. Identifying a new, rare or odd compound as being isoelectronic with an already known one offers clues to possible properties and reactions.
Examples
The N atom and the O+
radical ion are isoelectronic because each has five electrons in the outer electronic shell. Similarly, the cations K+
, Ca2+
, and Sc3+
and the anions Cl−
, S2−
, and P3−
are all isoelectronic with the Ar atom. In such monatomic cases, there is a clear trend in the sizes of such species, with atomic radius decreasing as charge increases.
CO, N
2 and NO+
are isoelectronic because each has two nuclei and 10 valence electrons, with each atom considered to have 5 of them (a lone-pair and a triple-bond). Isoelectronicity does not relate to formal charge on the atoms in a structure: these all have the same configuration even though carbon monoxide has formal charges that are balanced (−:C≡O:+) whereas dinitrogen has each atom neutral (:N≡N:) and nitrosonium has an overall net charge.
Isoelectronicity leads to the concept of hydrogen-like atoms, ions with one electron which are thus isoelectronic with hydrogen.
The uncharged H
2C=C=O (ethenone) molecule and the zwitterionic H
2C=N+
=N−
(diazomethane) molecule are isoelectronic.

CH
3COCH
3 (acetone) and CH
3N
2CH
3 (dimethyldiazene) are not isoelectronic. They do have the same number of nuclei and the same number of valence electrons, but the atoms' connectivity is different: the first one has both methyl (CH
3) groups attached to carbonyl's (CO's) carbon atom, forming a branched trigonal planar shape: H3C-C(=O)-CH3; the second molecule's structure has a consecutive attachment of the main atoms: H3C-N=N-CH3 and its methyl groups are not connected to the same nitrogen atom.
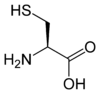
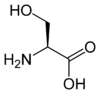
The amino acids tellurocysteine, selenocysteine, cysteine and serine are also considered (at least valence) isoelectronic.
References
- ↑ Isoelectronic Configurations iun.edu
- ↑ Isoelectronic thefreedictionary.com
- ↑ Advances in Organonmetallic chemistry. F.G.A. Stone page 190 google books link