Island of stability
| Nuclear physics |
|---|
 |
| Nucleus · Nucleons (p, n) · Nuclear force · Nuclear reaction |
|
Nuclear models and stability Liquid drop · Nuclear shell · Nuclear structure Binding energy · p–n ratio · Drip line · Stability Isl. |
|
Alpha α · Beta β (2β, β+) · K/L capture · Isomeric (Gamma γ · Internal conversion) · Spontaneous fission · Cluster decay · Neutron emission · Proton emission |
|
Nucleosynthesis topics Nuclear fusion Processes: Stellar · Big Bang · Supernova Nuclides: Primordial · Cosmogenic · Artificial |
|
Scientists |
In nuclear physics, the island of stability is a set of predicted, but as-yet undiscovered, heavier isotopes of transuranium elements which are theorized to be much more stable than some of those closer in atomic number to uranium. Specifically, they are expected to have radioactive decay half-lives of minutes or days, with "some optimists" expecting half-lives of millions of years.[1] Klaus Blaum expects the island of stability to occur in the region near 300Ubn.[2]
Theory and origin
One fact should be emphasized from the outset: while the various theoretical predictions about the superheavy nuclei differ as to the expected half-lives and regions of stability, all theoretical predictions are in agreement: superheavy nuclei can exist. Thus, the search for superheavy nuclei remains as a unique, rigorous test of the predictive power of modern theories of the structure of nuclei.
The possibility of an "island of stability" was first proposed by Glenn T. Seaborg in the late 1960s.[4] The hypothesis is based upon the nuclear shell model, which implies that the atomic nucleus is built up in "shells" in a manner similar to the structure of the much larger electron shells in atoms. In both cases, shells are just groups of quantum energy levels that are relatively close to each other. Energy levels from quantum states in two different shells will be separated by a relatively large energy gap. So when the number of neutrons and protons completely fills the energy levels of a given shell in the nucleus, the binding energy per nucleon will reach a local maximum and thus that particular configuration will have a longer lifetime than nearby isotopes that do not possess filled shells.[5]
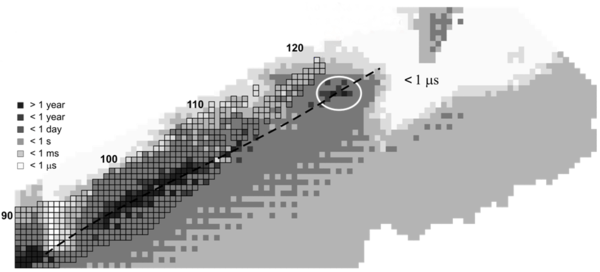
A filled shell would have "magic numbers" of neutrons and protons. One possible magic number of neutrons for spherical nuclei is 184, and some possible matching proton numbers are 114, 120 and 126 – which would mean that the most stable spherical isotopes would be flerovium-298, unbinilium-304 and unbihexium-310. Of particular note is Ubh-310, which would be "doubly magic" (both its proton number of 126 and neutron number of 184 are thought to be magic) and thus the most likely to have a very long half-life. (The next lighter doubly magic spherical nucleus is lead-208, the heaviest known stable nucleus and most stable heavy metal.)
Recent research indicates that large nuclei are deformed, causing magic numbers to shift. Hassium-270 is now believed to be a doubly magic deformed nucleus, with deformed magic numbers 108 and 162.[6][7] It has a half-life of 3.6 seconds.
Isotopes have been produced with enough protons to plant them upon an island of stability but with too few neutrons to even place them upon the island's outer "shores". It is possible that these elements possess unusual chemical properties and, if they have isotopes with adequate lifespans, would be available for various practical applications (such as particle accelerator targets and as neutron sources as well). In particular, the very small critical masses of transplutonic elements (possibly as small as grams) implies that if stable elements could be found, they would enable small and compact nuclear bombs either directly or by serving as primaries to help ignite fission/fusion secondaries; this possibility motivated much of the early research and multiple nuclear tests by the United States (including Operation Plowshare) and the Soviet Union aimed at producing such elements.[8]
Half-lives of the highest-numbered elements
All elements with an atomic number above 82 (lead) are unstable, and the "stability" (half-life of the longest-lived known isotope) of elements generally decreases with rising atomic numbers from the relatively stable uranium (92) upwards to the heaviest known element: 118. The longest-lived observed isotopes of each of the heaviest elements are shown in the following table.
(Note that for elements 108–118, the longest-lived known isotope is always the heaviest or second-heaviest (111, 115) one discovered, making it likely that there are still longer-lived isotopes among the undiscovered heavier ones)
For comparison, the shortest-lived element with atomic number below 100 is francium (element 87) with a half-life of 22 minutes.
The half-lives of nuclei in the island of stability itself are unknown since none of the isotopes that would be "on the island" have been observed. Many physicists think they are relatively short, on the order of minutes or days.[1] Some theoretical calculations indicate that their half-lives may be long, on the order of 109 years.[13]
The alpha-decay half-lives of 1700 nuclei with 100 ≤ Z ≤ 130 have been calculated in a quantum tunneling model with both experimental and theoretical alpha-decay Q-values.[14][15][16][17][18][19] The theoretical calculations are in good agreement with the available experimental data.
A possible stronger decay mode for the heaviest superheavies was shown to be cluster decay by Dorin N. Poenaru, R.A. Gherghescu, and Walter Greiner.[20]
Islands of relative stability
| Region of relative stability: radium-226 to einsteinium-252 | ||||||||||||||||||||||||||||||
| 88 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | |||||||||||||||||||
| 154 |
|
250Cm | 252Cf | 154 | ||||||||||||||||||||||||||
| 153 | 251Cf | 252Es | 153 | |||||||||||||||||||||||||||
| 152 | 248Cm | 250Cf | 152 | |||||||||||||||||||||||||||
| 151 | 247Cm | 248Bk | 249Cf | 151 | ||||||||||||||||||||||||||
| 150 | 244Pu | 246Cm | 247Bk | 150 | ||||||||||||||||||||||||||
| 149 | 245Cm | 149 | ||||||||||||||||||||||||||||
| 148 | 242Pu | 243Am | 244Cm | 148 | ||||||||||||||||||||||||||
| 147 | 241Pu | |
243Cm | 147 | ||||||||||||||||||||||||||
| 146 | 238U | 240Pu | 241Am | 146 | ||||||||||||||||||||||||||
| 145 | 239Pu | 145 | ||||||||||||||||||||||||||||
| 144 | 236U | 237Np | 238Pu | 144 | ||||||||||||||||||||||||||
| 143 | 235U | 236Np | 143 | |||||||||||||||||||||||||||
| 142 | 232Th | 234U | 235Np | 236Pu | 142 | |||||||||||||||||||||||||
| 141 | 233U | 141 | ||||||||||||||||||||||||||||
| 140 | 228Ra | 230Th | 231Pa | 232U |
|
140 | ||||||||||||||||||||||||
| 139 | 229Th | 139 | ||||||||||||||||||||||||||||
| 138 | 226Ra | 227Ac | 228Th | 138 | ||||||||||||||||||||||||||
| 88 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | |||||||||||||||||||
| Only isotopes with a half-life of at least one year are listed. | ||||||||||||||||||||||||||||||
232
Th (thorium), 235
U and 238
U (uranium) are the only naturally occurring isotopes beyond bismuth that are relatively stable over the current lifespan of the universe. Even bismuth was found to be slightly unstable in 2003, with an α-emission half-life of 1.9×1019 years for 209
Bi. All elements beyond bismuth have relatively or very unstable isotopes: astatine, radon, and francium are extremely short-lived (and only have half-lives longer than isotopes of the heaviest elements found so far). Even thorium, with the largest known half-life in this region (1.4×1010 years for 232
Th), is still about a billion times shorter than 209
Bi, so the main periodic table ends there.
By geographical analogy, bismuth is the shore edge of a continent. A continental shelf continues though, with shallows beginning at radium (see 'map' at right) that rapidly drop off again after californium. Significant islands appear at thorium and uranium, and with minor ones (i.e. plutonium and curium) form an archipelago. All of this is surrounded by a "sea of instability".[21]
Current theoretical investigation indicates that in the region Z = 106–108 and N ≈ 160–164, a small ‘island/peninsula’ might be stable with respect to fission and beta decay, such superheavy nuclei undergoing only alpha decay.[15][16][17] Also, 298
Fl is not the center of the magic island as predicted earlier.[22] On the contrary, the nucleus with Z = 110, N = 183 (293Ds) appears to be near the center of a possible 'magic island' (Z = 104–116, N ≈ 176–186). In the N ≈ 162 region the beta-stable, fission survived 268
Sg is predicted to have alpha-decay half-life ~3.2 hours that is greater than that (~28 s) of the deformed doubly-magic 270
Hs.[23] The superheavy nucleus 268
Sg has not been produced in the laboratory as yet (2009). For superheavy nuclei with Z > 116 and N ≈ 184 the alpha-decay half-lives are predicted to be less than one second. The nuclei with Z = 120, 124, 126 and N = 184 are predicted to form spherical doubly-magic nuclei and be stable with respect to fission.[24] Calculations in a quantum tunneling model show that such superheavy nuclei would undergo alpha decay within microseconds or less.[15][16][17]
Synthesis problems
The manufacture of nuclei on the island of stability proves to be very difficult because the nuclei available as starting materials do not deliver the necessary sum of neutrons. For the synthesis of isotope 298 of flerovium, one could use an isotope of plutonium and one of calcium that together have a sum of at least 298 nucleons; for example, calcium-50 and plutonium-248. These and heavier isotopes are not available in measurable quantities, making production virtually impossible with current methods. The same problem exists for the other possible combinations of isotopes needed to generate elements on the island using target-projectile methods. It may be possible to generate the isotope 298 of flerovium, if the multi-nucleon transfer reactions would work in low-energy collisions of actinide nuclei.[25] One of these reactions may be:
- 248Cm + 238U → 298Fl + 186W + 2 1
0n
Hypothetical second island
Recently, interest has been expressed over the possibility of a second island of stability. The idea of a second island of stability was presented by Yuri Oganessian at the 235th national meeting of the American Chemical Society. This new island would be centered around element 164 (unhexquadium), especially the isotope 482Uhq, with a stability similar to that of flerovium.[26] It is thought that to be able to synthesize these elements, a new, stronger particle accelerator would be needed.[27]
Concept criticism
Norman D. Cook notes the fact that despite the unanimity of [mainstream] theoretical models in predicting the existence of super-heavies, decades of searching for such isotopes, in both nature and through the collision of large nuclei, have come up empty. He argues that this is because the island is "a mirage" due to improper "extrapolation from conventional ideas about the nuclear force".[13] Cook advocates for a nuclear structure model that predicts decreasing stability in the super-heavy region.[28]
See also
References
- ↑ 1.0 1.1 "Superheavy Element 114 Confirmed: A Stepping Stone to the Island of Stability". Retrieved 11 October 2009.
- ↑ "Superheavy, and yet stable". Max Planck Institute for Nuclear Physics. 23 August 2012. Retrieved 23 June 2013.
Quoting: “We expect [the island of stability] at around element 120,” says Blaum, “and to be more precise, in a nucleus with around 180 neutrons.” - ↑ Seaborg, G. T. (1987). "Superheavy elements". Contemporary Physics 28: 33. doi:10.1080/00107518708211038.
- ↑ "The Island of Stability?". Retrieved 2012-07-24.
- ↑ "Shell Model of Nucleus". HyperPhysics. Department of Physics and Astronomy, Georgia State University. Retrieved 22 January 2007.
- ↑ Dvořák, Jan (2007-07-12). "PhD. Thesis: Decay properties of nuclei close to Z = 108 and N = 162". Technische Universität München.
- ↑ Dvorak, J.; Brüchle, W.; Chelnokov, M.; Dressler, R.; Düllmann, Ch.; Eberhardt, K.; Gorshkov, V.; Jäger, E. et al. (2006). "Doubly Magic Nucleus Hs162-108-270". Physical Review Letters 97 (24): 242501. Bibcode:2006PhRvL..97x2501D. doi:10.1103/PhysRevLett.97.242501. PMID 17280272.
- ↑ pp. 129–133, The physical principles of thermonuclear explosives, inertial confinement fusion, and the quest for fourth generation nuclear weapons (Andre Gsponer and Jean-Pierre Hurni 2009)
- ↑ Emsley, John (2001). Nature's Building Blocks ((Hardcover, First Edition) ed.). Oxford University Press. pp. (pages 143, 144,458). ISBN 0-19-850340-7.
- ↑ Khuyagbaatar, J. (2014). "Ca48+Bk249 Fusion Reaction Leading to Element Z=117: Long-Lived α-Decaying Db270 and Discovery of Lr266". Phys. Rev. Lett. 112: 172501. Bibcode:2014PhRvL.112b2501S. doi:10.1103/PhysRevLett.112.022501. Retrieved May 8, 2014.
- ↑ Alexandra Witze (April 6, 2010). "Superheavy element 117 makes debut". Retrieved April 6, 2010.
- ↑ "Hassium". Rsc.org. Retrieved 2014-05-02.
- ↑ 13.0 13.1 Cook, N. D. (2010). "Models of the Atomic Nucleus". Springer. doi:10.1007/978-3-642-14737-1. ISBN 978-3-642-14736-4. Available to freely download as a PDF from the author's website:
- ↑ P. Roy Chowdhury, C. Samanta, and D. N. Basu (January 26, 2006). "α decay half-lives of new superheavy elements". Phys. Rev. C 73: 014612. arXiv:nucl-th/0507054. Bibcode:2006PhRvC..73a4612C. doi:10.1103/PhysRevC.73.014612.
- ↑ 15.0 15.1 15.2 C. Samanta, P. Roy Chowdhury and D.N. Basu (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nucl. Phys. A 789: 142–154. arXiv:nucl-th/0703086. Bibcode:2007NuPhA.789..142S. doi:10.1016/j.nuclphysa.2007.04.001.
- ↑ 16.0 16.1 16.2 P. Roy Chowdhury, C. Samanta, and D. N. Basu (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Phys. Rev. C 77 (4): 044603. arXiv:0802.3837. Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603.
- ↑ 17.0 17.1 17.2 P. Roy Chowdhury, C. Samanta, and D. N. Basu (2008). "Nuclear half-lives for α-radioactivity of elements with 100 < Z < 130". At. Data & Nucl. Data Tables 94 (6): 781. arXiv:0802.4161. Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
- ↑ P. Roy Chowdhury, D. N. Basu and C. Samanta (January 26, 2007). "α decay chains from element 113". Phys. Rev. C 75 (4): 047306. arXiv:0704.3927. Bibcode:2007PhRvC..75d7306C. doi:10.1103/PhysRevC.75.047306.
- ↑ Chhanda Samanta, Devasish Narayan Basu, and Partha Roy Chowdhury (2007). "Quantum tunneling in 277112 and its alpha-decay chain". Journal of the Physical Society of Japan 76 (12): 124201–124204. arXiv:0708.4355. Bibcode:2007JPSJ...76l4201S. doi:10.1143/JPSJ.76.124201.
- ↑ Dorin N Poenaru, R.A. Gherghescu, Walter Greiner (2011). "Heavy-Particle Radioactivity of Superheavy Nuclei". Phys. Rev. Lett. 107 (6): 062503. arXiv:1106.3271. Bibcode:2011PhRvL.107f2503P. doi:10.1103/PhysRevLett.107.062503. PMID 21902317.
- ↑ Note graphic: Known and predicted regions of nuclear stability, surrounded by a “sea” of instability. cf. the Chart of Nuclides by half-life.
- ↑ Sven Gösta Nilsson, Chin Fu Tsang, Adam Sobiczewski, Zdzislaw Szymaski, Slawomir Wycech, Christer Gustafson, Inger-Lena Lamm, Peter Möller and Björn Nilsson (February 14, 1969). "On the nuclear structure and stability of heavy and superheavy elements". Nuclear Physics A 131 (1): 1–66. Bibcode:1969NuPhA.131....1N. doi:10.1016/0375-9474(69)90809-4.
- ↑ J. Dvorak, W. Brüchle, M. Chelnokov, R. Dressler, Ch. E. Düllmann, K. Eberhardt, V. Gorshkov, E. Jäger, R. Krücken, A. Kuznetsov, Y. Nagame, F. Nebel,1 Z. Novackova, Z. Qin, M. Schädel, B. Schausten, E. Schimpf, A. Semchenkov, P. Thörle, A. Türler, M. Wegrzecki, B. Wierczinski, A. Yakushev, and A. Yeremin (2006). "Doubly Magic Nucleus 270108 Hs-162". Physical Review Letters 97 (24): 242501. Bibcode:2006PhRvL..97x2501D. doi:10.1103/PhysRevLett.97.242501. PMID 17280272.
- ↑ S. Cwiok, P.-H. Heenen and W. Nazarewicz (2005). "Shape coexistence and triaxiality in the superheavy nuclei" (PDF). Nature 433 (7027): 705–9. Bibcode:2005Natur.433..705C. doi:10.1038/nature03336. PMID 15716943.
- ↑ Zagebraev, V; Greiner, W (2008). "Synthesis of superheavy nuclei: A search for new production reactions". Physical Review C 78 (3): 034610. arXiv:0807.2537. Bibcode:2008PhRvC..78c4610Z. doi:10.1103/PhysRevC.78.034610.
- ↑ http://link.springer.com/article/10.1007%2FBF01406719/lookinside/000.png
- ↑ "Nuclear scientists eye future landfall on a second 'island of stability'". Eurekalert.org. 2008-04-06. Retrieved 2014-05-02.
- ↑ Cook, N. D. (1990). "The Attenuation of the Periodic Table". Modern Physics Letters A 05 (17): 1321. doi:10.1142/S0217732390001505.
External links
- Six new isotopes of the superheavy elements discovered (Oct 26 2010, Physorg news. Inc chart of heavy nuclides)
- Exploring the island of superheavy elements (April 2010, re decay of 117; with chart)
- Hunting the biggest atoms in the universe (July 23, 2008; claimed finding natural atoms of 122 protons and 170 neutrons)
- The hunt for superheavy elements (April 7, 2008; prediction of seaborgium-290 half-life of 108 years.)
- Here be stability (Nature Aug 2006 with JINR diagram of heavy nuclides and predicted IoS)
- Superheavy elements (Jul 2004 Yuri Oganessian of JINR )
- Uut and Uup Add Their Atomic Mass to Periodic Table (Feb 2004)
- Can superheavy elements (such as Z = 116 or 118) be formed in a supernova? Can we observe them? 2004 – "maybe"
- Second postcard from the island of stability (Oct 2001) nuclides with 116 protons and mass 292
- The synthesis of spherical superheavy nuclei in 48Ca induced reactions Reports the 1999 synthesis of Z = 114, N + Z = 287.
- New elements discovered and the island of stability sighted (Aug 1999; includes report on article later retracted)
- First postcard from the island of nuclear stability (1999; first few Z = 114 atoms)
- NOVA: Island of Stability (2006. 13m TV segment, with transcript)
- New York Times editorial by Oliver Sacks regarding the Island of Stability theory (Feb 2004 re 113 and 115)
- Tendency equation and curve of stable nuclides
- Ununoctium Applications (diagram of IoS)