Isavuconazole
 | |
| Systematic (IUPAC) name | |
|---|---|
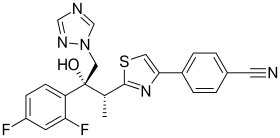
| 4-{2-[(1R,2R)-(2,5-Difluorophenyl)-2-hydroxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]-1,3-thiazol-4-yl}benzonitrile | |
| Clinical data | |
| Trade names | Cresemba (prodrug form) |
| |
| Oral; Intravenous | |
| Identifiers | |
|
| |
| None | |
| PubChem | CID 6918485 |
| ChemSpider |
5293682 |
| UNII |
60UTO373KE |
| ChEMBL |
CHEMBL409153 |
| NIAID ChemDB | 416566 |
| Chemical data | |
| Formula | C22H17F2N5OS |
| 437.47 g/mol | |
|
SMILES
| |
| |
| | |
Isavuconazole (BAL4815; trade name Cresemba) is a triazole antifungal drug. Its prodrug, isavuconazonium sulfate (BAL8557), was granted approval by the U.S. Food and Drug Administration (FDA) on March 6, 2015[1]
During its Phase III drug trials, Astellas partnered with Basilea Pharmaceutica, the developer of the drug, for rights to co-development and marketing of isavuconazole. [2]
On May 28, 2013, Basilea Pharmaceutica announced it had been granted orphan drug status by the FDA for treatment of aspergillosis.[3] Since then, it has also been granted orphan drug status for the treatment of invasive candidiasis.[4]
Mechanism of Action
Isavuconazole works by inhibiting lanosterol 14 alpha-demethylase, the enzyme responsible for converting lanosterol to ergosterol by demethylation. The resulting depletion of ergosterol and build up of lanosterol compromise the structure of the fungal cell membrane. Mammalian cells are resistant to demethylation inhibition by azoles, making the drug effects specific to fungi.[5]
Indications for Use
Isovuconazole has been approved by the FDA for treatment of invasive aspergillosis and invasive mucormycosis in adults ages 18 years and older. Both infections are caused by mold and fungi common to the environment, and occur in individuals who are immunosuppressed or have other complicating conditions, such as diabetes or lung disease.[6][7]
Dosing
The intravenous (IV) and oral formulations of isovuconazole are bioequivalent, so formulations may be switched between without an additional loading dose.
Side Effects
Common
- Cardiovascular: peripheral edema
- Endocrine: hypokalemia
- Gastrointestinal: constipation, diarrhea, nausea, vomiting
- Musculoskeletal: backache
- Neurologic: headache
- Respiratory: cough
Rare
- Hepatic: cholestasis, hepatitis, increased liver function tests, liver failure
- Immunologic: hypersensitivity reaction
- Renal: renal failure
- Respiratory: acute respiratory failure
- Other: infusion reaction (IV only)[8]
Warnings
Isavuconazole should not be administered with other medications that strongly inhibit or induce the enzyme CYP3A4 due to the effect on plasma concentration. CYP3A4 inducers can result in subtherapeutic drug levels, and CYP3A4 inhibitors can cause supratherapeutic levels with increased adverse events and toxicity.
Studies have shown isavuconazole to have a dose dependent effect of shortening the QTc interval. It is contraindicated for use in individuals with familial short QT syndrome. Additive effects with other drugs that shorten the QTc have not been evaluated.
Clinical Trials
Isovuconazole was demonstrated in Phase III clinical trial SECURE to be non-inferior to voriconazole, another triazole antifungal, in the treatment of invasive aspergillosis, with all cause mortality at 18.6%, compared to 20.2% in the comparator group. It additionally demonstrated a similar side effect profile.
Data from the VITAL study showed that isavuconazole could be used in treatment of invasive mucormycosis, but did not evaluate its clinical efficacy for this indication.
References
- ↑
- ↑ Saboo, Alok. "Basilea Announces Global Partnership With Astellas for Its Antifungal Isavuconazole." FierceBiotech. N.p., 24 Feb. 2010. Web.
- ↑ "Basilea reports isavuconazole orphan drug designation by U.S. FDA." Market Wired. 28 May 2013.
- ↑ "FDA Grants Orphan Drug Designation to Astellas for Isavuconazole for the Treatment of Invasive Candidiasis." News Releases. Astellas. 3 Nov 2014.
- ↑ Cresemba (isovuconazole sulfate) [prescribing information]. Astella Pharma US, Inc. Revised March 2015.
- ↑ "Aspergillosis." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 08 Sept. 2014.
- ↑ "Astellas Receives FDA Approval for CRESEMBA® (isavuconazonium Sulfate) for the Treatment of Invasive Aspergillosis and Invasive Mucormycosis." PR Newswire. N.p., 6 Mar. 2015.
- ↑ "Isavuconazonium." Micromedex Solutions. Truven Health Analytics, n.d. Web. <www.micromedexsolutions.com>.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||