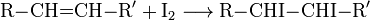Iodine value
The iodine value (or "iodine adsorption value" or "iodine number" or "iodine index") in chemistry is the mass of iodine in grams that is consumed by 100 grams of a chemical substance. Iodine numbers are often used to determine the amount of unsaturation in fatty acids. This unsaturation is in the form of double bonds, which react with iodine compounds. The higher the iodine number, the more C=C bonds are present in the fat.[1] It can be seen from the table that coconut oil is very saturated, which means it is good for making soap. On the other hand, linseed oil is highly unsaturated, which makes it a drying oil, well suited for making oil paints.
Table of iodine values
| Fat | Iodine number[1] |
|---|---|
| Tung oil | 163 – 173 |
| Grape seed oil | 124 – 143 |
| Palm oil | 44 – 51 |
| Olive oil | 80 – 88 |
| Coconut oil | 7 – 12 |
| Palm kernel oil | 16 – 19 |
| Cocoa butter | 35 – 40 |
| Jojoba oil | 80 ~80 |
| Poppyseed oil | 133 ~133 |
| Cottonseed oil | 100 – 117 |
| Corn oil | 109 – 133 |
| Wheat germ oil[2] | 115 – 134 |
| Sunflower oil | 125 – 144 |
| Linseed oil | 136 – 178 |
| Soybean oil | 120 – 136 |
| Peanut oil | 84 – 105 |
| Rice bran oil | 99 – 108 |
| Walnut oil[3] | 120 – 140 |
Methodology
This particular analysis is an example of iodometry. A solution of iodine is yellow/brown in color. When this is added to a solution to be tested, however, any chemical group (usually in this test C=C double bonds) that react with iodine effectively reduce the strength, or magnitude of the colour (by taking iodine out of solution). Thus the amount of iodine required to make a solution retain the characteristic yellow/brown colour can effectively be used to determine the amount of iodine sensitive groups present in the solution.
The chemical reaction associated with this method of analysis involves formation of the diiodo alkane (R and R' symbolize alkyl or other organic groups):
The precursor alkene (RCH=CHR') is colourless and so is the organoiodine product (RCHI-CHIR').
In a typical procedure, the fatty acid is treated with an excess of the Hanuš or Wijs solutions, which are, respectively, solutions of iodine monobromide (IBr) and iodine monochloride (ICl) in glacial acetic acid. Unreacted iodine monobromide (or monochloride) is then allowed to react with potassium iodide, converting it to iodine, whose concentration can be determined by titration with sodium thiosulfate.[4]
Related methods of analysis
- Saponification value
- Acid number
- Bromine number
- Hydroxyl value
References
- ↑ 1.0 1.1 Thomas, Alfred (2002). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_173.
- ↑ http://online.personalcarecouncil.org/ctfa-static/online/lists/cir-pdfs/pr248.pdf
- ↑ http://thesoapdish.com/oil-properties-chart.htm
- ↑ Firestone D (May–Jun 1994). "Determination of the iodine value of oils and fats: summary of collaborative study". J AOAC Int. 77 (3): 674–6. PMID 8012219.