Interleukin 4
| Interleukin 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 analysis of the solution structure of human interleukin 4 determined by heteronuclear three-dimensional nuclear magnetic resonance techniques | |||||||||
| Identifiers | |||||||||
| Symbol | IL4 | ||||||||
| Pfam | PF00727 | ||||||||
| Pfam clan | CL0053 | ||||||||
| InterPro | IPR002354 | ||||||||
| PROSITE | PDOC00655 | ||||||||
| SCOP | 2int | ||||||||
| SUPERFAMILY | 2int | ||||||||
| |||||||||
The interleukin 4 (IL4) is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. The cell that initially produces IL-4, thus inducing Th0 differentiation, has not been identified, but recent studies suggest that basophils may be the effector cell.[1] It is closely related and has functions similar to Interleukin 13.
Function
It has many biological roles, including the stimulation of activated B-cell and T-cell proliferation, and the differentiation of B cells into plasma cells. It is a key regulator in humoral and adaptive immunity. IL-4 induces B-cell class switching to IgE, and up-regulates MHC class II production. IL-4 decreases the production of Th1 cells, macrophages, IFN-gamma, and dendritic cell IL-12.
Overproduction of IL-4 is associated with allergies.[2]
Inflammation and wound repair
Tissue macrophages play an important role in chronic inflammation and wound repair. The presence of IL-4 in extravascular tissues promotes alternative activation of macrophages into M2 cells and inhibits classical activation of macrophages into M1 cells. An increase in repair macrophages (M2) is coupled with secretion of IL-10 and TGF-β that result in a diminution of pathological inflammation. Release of arginase, proline, polyaminases and TGF-β by the activated M2 cell is tied with wound repair and fibrosis.[3]
Receptor
The receptor for Interleukin-4 is known as the IL-4Rα. This receptor exists in 3 different complexes throughout the body. Type 1 receptors are composed of the IL-4Rα subunit with a common γ chain and specifically bind IL-4. Type 2 receptors consist of an IL-4Rα subunit bound to a different subunit known as IL-13Rα1. These type 2 receptors have the ability to bind both IL-4 and IL-13, two cytokines with closely related biological functions.[4][5]
Structure
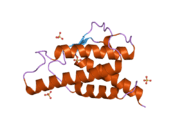
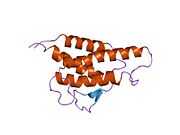
IL-4 has a compact, globular fold (similar to other cytokines), stabilised by 3 disulphide bonds.[6] One half of the structure is dominated by a 4 alpha-helix bundle with a left-handed twist.[7] The helices are anti-parallel, with 2 overhand connections, which fall into a 2-stranded anti-parallel beta-sheet.[7]
Discovery
This cytokine was co-discovered by Maureen Howard and William Paul[8] and by Dr. Ellen Vitetta and her research group in 1982.
The nucleotide sequence for human IL-4 was isolated four years later confirming its similarity to a mouse protein called B-cell stimulatory factor-1 (BCSF-1).[9]
Clinical significance
IL-4 also has been shown to drive mitogenesis, dedifferentiation, and metastasis in rhabdomyosarcoma.[10] IL-4, along with other Th2 cytokines, is involved in the airway inflammation observed in the lungs of patients with allergic asthma.
See also
References
- ↑ Sokol, C.L., Barton, G.M., Farr, A.G. & Medzhitov, R. (2008). "A mechanism for the initiation of allergen-induced T helper type 2 responses". Nat Immunol 9 (3): 310–318. doi:10.1038/ni1558. PMID 18300366.
- ↑ Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA (December 1997). "The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor". N. Engl. J. Med. 337 (24): 1720–5. doi:10.1056/NEJM199712113372403. PMID 9392697. Lay summary – eurekalert.org.
- ↑ Jon Aster, Vinay Kumar, Abul K. Abbas; Nelson Fausto (2009). Robbins & Cotran Pathologic Basis of Disease (8th ed.). Philadelphia: Saunders. p. 54. ISBN 1-4160-3121-9.
- ↑ Maes T, Joos GF, Brusselle GG (September 2012). "Targeting interleukin-4 in asthma: lost in translation?". Am. J. Respir. Cell Mol. Biol. 47 (3): 261–70. doi:10.1165/rcmb.2012-0080TR. PMID 22538865.
- ↑ Chatila TA (October 2004). "Interleukin-4 receptor signaling pathways in asthma pathogenesis". Trends Mol Med 10 (10): 493–9. doi:10.1016/j.molmed.2004.08.004. PMID 15464449.
- ↑ Carr C, Aykent S, Kimack NM, Levine AD (February 1991). "Disulfide assignments in recombinant mouse and human interleukin 4". Biochemistry 30 (6): 1515–23. doi:10.1021/bi00220a011. PMID 1993171.
- ↑ 7.0 7.1 Walter MR, Cook WJ, Zhao BG, Cameron RP, Ealick SE, Walter RL, Reichert P, Nagabhushan TL, Trotta PP, Bugg CE (October 1992). "Crystal structure of recombinant human interleukin-4". J. Biol. Chem. 267 (28): 20371–6. PMID 1400355.
- ↑ Howard M, Paul WE (1982). "Interleukins for B lymphocytes". Lymphokine Res. 1 (1): 1–4. PMID 6985399.
- ↑ Yokota T et al. (1986). "Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities". Proc. Natl. Acad. Sci. U.S.A. 83 (16): 5894–8. doi:10.1073/pnas.83.16.5894. PMC 386403. PMID 3016727.
- ↑ Hosoyama T, Aslam MI, Abraham J, Prajapati SI, Nishijo K, Michalek JE, Zarzabal LA, Nelon LD, Guttridge DC, Rubin BP, Keller C (May 2011). "IL-4R Drives Dedifferentiation, Mitogenesis, and Metastasis in Rhabdomyosarcoma". Clin Cancer Res 17 (9): 2757–2766. doi:10.1158/1078-0432.CCR-10-3445. PMC 3087179. PMID 21536546.
Further reading
- Apte SH, Baz A, Kelso A, Kienzle N (2008). "Interferon-gamma and interleukin-4 reciprocally regulate CD8 expression in CD8+ T cells". Proc Natl Acad Sci U S A. 105 (45): 17475–80. doi:10.1073/pnas.0809549105. PMC 2580749. PMID 18988742.
- Kay AB, Barata L, Meng Q et al. (1997). "Eosinophils and eosinophil-associated cytokines in allergic inflammation". Int. Arch. Allergy Immunol. 113 (1–3): 196–9. doi:10.1159/000237545. PMID 9130521.
- Marone G, Florio G, Petraroli A, de Paulis A (2001). "Dysregulation of the IgE/Fc epsilon RI network in HIV-1 infection". J. Allergy Clin. Immunol. 107 (1): 22–30. doi:10.1067/mai.2001.111589. PMID 11149986.
- Marone G, Florio G, Triggiani M et al. (2001). "Mechanisms of IgE elevation in HIV-1 infection". Crit. Rev. Immunol. 20 (6): 477–96. doi:10.1615/critrevimmunol.v20.i6.40. PMID 11396683.
- Maeda S, Yanagihara Y (2001). "[Inflammatory cytokines (IL-4, IL-5 and IL-13)]". Nippon Rinsho 59 (10): 1894–9. PMID 11676128.
- Izuhara K, Arima K, Yasunaga S (2003). "IL-4 and IL-13: their pathological roles in allergic diseases and their potential in developing new therapies". Current drug targets. Inflammation and allergy 1 (3): 263–9. doi:10.2174/1568010023344661. PMID 14561191.
- Copeland KF (2006). "Modulation of HIV-1 transcription by cytokines and chemokines". Mini reviews in medicinal chemistry 5 (12): 1093–101. doi:10.2174/138955705774933383. PMID 16375755.
- Olver S, Apte S, Baz A, Kienzle N (2007). "The duplicitous effects of interleukin 4 on tumour immunity: how can the same cytokine improve or impair control of tumour growth?". Tissue Antigens 69 (4): 293–8. doi:10.1111/j.1399-0039.2007.00831.x. PMID 17389011.
- Sokol CL, Chu NQ, Shuang Yu, Simone Nish, Terri Laufer & Ruslan Medzhitov (2009). "Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response". Nature Immunology 10 (7): 713–720. doi:10.1038/ni.1738. PMC 3252751. PMID 19465907.
- Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R (July 2009). "Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response". Nat. Immunol. 10 (7): 713–20. doi:10.1038/ni.1738. PMC 3252751. PMID 19465907.
External links
- Interleukin-4 at the US National Library of Medicine Medical Subject Headings (MeSH)
- Interleukin-4 from Gentaur at Gentaur
- Recombinant Human Interleukin-4 from Cornell University
- Interleukin-4 from Allergy Glossary at Health On the Net Foundation
| |||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article incorporates text from the public domain Pfam and InterPro IPR002354