Incremental cost-effectiveness ratio
The incremental cost-effectiveness ratio (ICER) is a statistic used in cost-effectiveness analysis to summarise the cost-effectiveness of a health care intervention. It is defined by the difference in cost between two possible interventions, divided by the difference in their effect. It represents the average incremental cost associated with 1 additional unit of the measure of effect. The ICER can be estimated as:
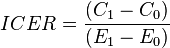 ,
,
where 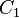 and
and 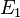 are the cost and effect in the intervention group and where
are the cost and effect in the intervention group and where 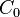 and
and 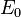 are the cost and effect in the control care group.[1] Costs are usually described in monetary units, while effects can be measured in terms of health status or another outcome of interest. A common application of the ICER is in cost-utility analysis, in which case the ICER is synonymous with the cost per quality-adjusted life year (QALY) gained.
are the cost and effect in the control care group.[1] Costs are usually described in monetary units, while effects can be measured in terms of health status or another outcome of interest. A common application of the ICER is in cost-utility analysis, in which case the ICER is synonymous with the cost per quality-adjusted life year (QALY) gained.
Use as a decision rule
The ICER can be used as a decision rule in resource allocation. If a decision-maker is able to establish a willingness-to-pay value for the outcome of interest, it is possible to adopt this value as a threshold. If for a given intervention the ICER is above this threshold it will be deemed too expensive and thus should not be funded, whereas if the ICER lies below the threshold the intervention can be judged cost-effective. This approach has to some extent been adopted in relation to QALYs; for example, the National Institute for Health and Care Excellence adopts a nominal cost-per-QALY threshold of £20,000 to £30,000.[2] As such, the ICER facilitates comparison of interventions across various disease states and treatments. The use of ICERs therefore provides an opportunity to help contain health care costs without adverse health consequences.[3] They also provide to policy makers information on where resources should be allocated when they are limited.[4] As health care costs have continued to rise, many new clinical trials are attempting to integrate ICER into results to provide more evidence of potential benefit.[5]
Controversies
Many people feel that basing health care interventions on cost-effectiveness is a type of health care rationing and have expressed concern that using ICER will limit the amount or types of treatments and interventions available to patients.[4] Currently, the National Institute for Health and Care Excellence (NICE) of England’s National Health Service (NHS) uses cost-effectiveness studies to determine if new treatments or therapies provide better value relative to the treatment that is currently in use. With the number of cost-effectiveness studies rising, it is expected for a cost-effectiveness ratio threshold to be established for the acceptance of reimbursement or formulary listing. However, there is currently no evidence that health care systems have determined such a threshold;[6] without such a standard, the interpretation of ICER analyses may not be uniform.
The concern that ICER may lead to rationing has affected policy makers in the United States. The Patient Protection and Affordable Care Act of 2010 provided for the creation of the independent Patient-Centered Outcomes Research Institute (PCORI). The Senate Finance Committee in writing PPACA forbade PCORI from using “dollars-per-quality adjusted life year (or similar measure that discounts the value of a life because of an individual’s disability) as a threshold to establish what type of health care is cost effective or recommended.”[7]
References
- ↑ What is the incremental cost-effectiveness ratio (ICER)? GaBI Online. . Accessed 20 March 2012.
- ↑ Appleby, John; Devlin, Nancy; Parkin, David (2007). "NICE's cost effectiveness threshold". BMJ. doi:10.1136/bmj.39308.560069.BE. PMID 17717337.
- ↑ Orszag PR, Ellis P. Addressing rising health care costs—A view from the Congressional Budget Office. N Engl J Med, 2007; 357:1885–1887.
- ↑ 4.0 4.1 Cost-effective Medical Treatment: Putting an Updated Dollar Value on Human Life. Knowledge@Wharton, 30 April 2008. . Accessed 20 March 2012.
- ↑ Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, Cook J, Glick H, Liljas B, Petitti D, Reed S. Good research practices for cost-effectiveness analysis alongside clinical trials: The ISPOR RCT-CEA task force report. Value in Health, 2005; 8(5):521-533.
- ↑ Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: How are cost-effectiveness thresholds expected to emerge? Value in Health, 2004; 7(5):518-528.
- ↑ Wilkerson J. PCORI head vows not to do cost-effectiveness studies, but notes gray areas. InsideHealthPolicy.com, 28 September 2011. Accessed 20 March 2012.
| ||||||||||||||||||||||