Immunoglobulin E


Immunoglobulin E (IgE) is a kind of antibody (or immunoglobulin (Ig) "isotype") that has only been found in mammals. Monomers of IgE consist of two heavy chains (ε chain) and two light chains, with the ε chain containing 4 Ig-like constant domains (Cε1-Cε4).[1] IgE's main function is immunity to parasites such as helminths[2] like Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica.[3][4][5] IgE is utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum.[6]
IgE also has an essential role in type I hypersensitivity,[7] which manifests various allergic diseases, such as allergic asthma, most types of sinusitis, allergic rhinitis, food allergies, and specific types of chronic urticaria and atopic dermatitis. IgE also plays a pivotal role in allergic responses, such as anaphylactic reactions to drugs, bee stings, and antigen preparations used in desensitization immunotherapy.
Although IgE is typically the least abundant isotype—blood serum IgE levels in a normal ("non-atopic") individual are only 0.05% of the Ig concentration,[8] compared to 75% for the IgGs at 10 mg/ml, which are the isotypes responsible for most of the classical adaptive immune response—it is capable of triggering the most powerful inflammatory reactions.
IgE was simultaneously discovered in 1966 by two groups: Dr. Lawrence Lichtenstein[9] and Dr. Philip Norman in the Johns Hopkins Department of Medicine's Division of Allergy and Infectious Diseases, as well as Dr. Kimishige Ishizaka and Dr. Margaret M. Hornbrook[10] in the Children's Asthma Research Institute and Hospital in Denver, CO.[11]
Receptors
IgE primes the IgE-mediated allergic response by binding to Fc receptors found on the surface of mast cells and basophils. Fc receptors are also found on eosinophils, monocytes, macrophages and platelets in humans. There are two types of Fcε receptors:
- FcεRI (type I Fcε receptor), the high-affinity IgE receptor
- FcεRII (type II Fcε receptor), also known as CD23, the low-affinity IgE receptor
IgE can upregulate the expression of both types of Fcε receptors. FcεRI is expressed on mast cells, basophils, and the antigen-presenting dendritic cells in both mice and humans. Binding of antigens to IgE already bound by the FcεRI on mast cells causes cross-linking of the bound IgE and the aggregation of the underlying FcεRI, leading to the degranulation and the release of mediators from the cells. Basophils, upon the cross-linking of their surface IgE by antigens, release type 2 cytokines like interleukin-4 (IL-4) and interleukin-13 (IL-13) and other inflammatory mediators. The low-affinity receptor (FcεRII) is always expressed on B cells, but its expression can be induced on the surfaces of macrophages, eosinophils, platelets, and some T cells by IL-4.[12][13]
Function
There is much speculation into what physiological benefits IgE contributes, and, so far, circumstantial evidence in animal models and statistical population trends have hinted that IgE may be beneficial in fighting gut parasites such as Schistosoma mansoni, but this has not been conclusively proven in humans. Epidemiological research shows that IgE level is increased when infected by Schistosoma mansoni,[14] Necator americanus,[15] and nematodes[16] in human. It is most likely beneficial in removal of hookworms from the lung.
Although it is not yet well understood, IgE may play an important role in the immune system’s recognition of cancer,[17] in which the stimulation of a strong cytotoxic response against cells displaying only small amounts of early cancer markers would be beneficial. If this were the case, anti-IgE treatments such as omalizumab (for allergies) might have some undesirable side effects. However, a recent study, which was performed based on pooled analysis using comprehensive data from 67 phase I to IV clinical trials of omalizumab in various indications, concluded that a causal relationship between omalizumab therapy and malignancy is unlikely.[18]
Role in disease
Atopic individuals can have up to 10 times the normal level of IgE in their blood (as do sufferers of hyper-IgE syndrome). However, this may not be a requirement for symptoms to occur as has been seen in asthmatics with normal IgE levels in their blood - recent research has shown that IgE production can occur locally in the nasal mucosa.[19]
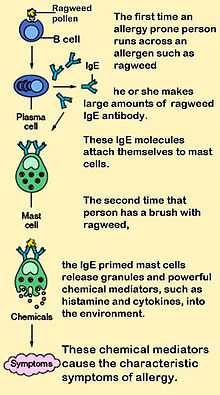
IgE that can specifically recognise an "allergen" (typically this is a protein, such as dust mite Der p 1, cat Fel d 1, grass or ragweed pollen, etc.) has a unique long-lived interaction with its high-affinity receptor FcεRI so that basophils and mast cells, capable of mediating inflammatory reactions, become "primed", ready to release chemicals like histamine, leukotrienes, and certain interleukins. These chemicals cause many of the symptoms we associate with allergy, such as airway constriction in asthma, local inflammation in eczema, increased mucus secretion in allergic rhinitis, and increased vascular permeability, it is presumed, to allow other immune cells to gain access to tissues, but which can lead to a potentially fatal drop in blood pressure as in anaphylaxis. Although the mechanisms of each response are fairly well understood, why some allergics develop such drastic sensitivities when others merely get a runny nose is still one of science's hot topics.
IgE is known to be elevated in various autoimmune disorders such as Lupus(SLE), Rheumatoid Arthritis(RA) & psoriasis, and is theorized to be of pathogenetic importance in RA and SLE by eliciting a hypersensitivity reaction.[20][21]
Regulation of IgE levels through control of B cell differentiation to antibody-secreting plasma cells is thought to involve the "low-affinity" receptor FcεRII, or CD23.[22] CD23 may also allow facilitated antigen presentation, an IgE-dependent mechanism whereby B cells expressing CD23 are able to present allergen to (and stimulate) specific T helper cells, causing the perpetuation of a Th2 response, one of the hallmarks of which is the production of more antibodies.[23]
Role in diagnosis
Diagnosis of allergy is most often done when a physician reviews a patient's history and finds a positive result for the presence of allergen specific IgE when conducting a skin or blood test.[24] Specific IgE testing is the proven test for allergy detection; evidence does not show that indiscriminate IgE testing or testing for immunoglobulin G (IgG) can support allergy diagnosis.[25]
Drugs targeting the IgE pathway
Currently, allergic diseases and asthma are usually treated with one or more of the following drugs: (1) antihistamines and antileukotrienes, which antagonize the inflammatory mediators histamine and leukotrienes, (2) local or systemic (oral or injectable) corticosteroids, which suppress a broad spectrum of inflammatory mechanisms, and (3) short or long-acting bronchodilators, which relax smooth muscle of constricted airway in asthma. Long-term uses of systemic corticosteroids are known to cause many serious side effects and are advisable to avoid, if alternative therapies are available.
IgE, the IgE synthesis pathway, and the IgE-mediated allergic/inflammatory pathway are all important targets in intervening with the pathological processes of allergy, asthma, and other IgE-mediated diseases. The B lymphocyte differentiation and maturation pathway that eventually generate IgE-secreting plasma cells go through the intermediate steps of IgE-expressing B lymphoblasts and involves the interaction with IgE-expressing memory B cells. Tanox, a biotech company based in Houston, Texas, proposed in 1987 that by targeting membrane-bound IgE (mIgE) on B lymphoblast and memory B cells, those cells can be lysed or down-regulated, thus achieving the inhibition of the production of antigen-specific IgE and hence a shift of immune balance toward non-IgE mechanisms.[26] Two approaches targeting the IgE pathway were evolved and both are in active development. In the first approach, the anti-IgE antibody drug omalizumab (trade name Xolair) recognises IgE not bound to its receptors and is used to neutralise or mop-up existing IgE and prevent it from binding to the receptors on mast cells and basophils. Xolair has been approved in many countries for treating severe, persistent allergic asthma. It has also been approved in March 2014 in the European Union[27] and the U. S.[28] for treating chronic spontaneous urticaria, which cannot be adequately treated with H1-antihistamines. In the second approach, antibodies specific for a domain of 52 amino acid residues, referred to as CεmX or M1’ (M1 prime), present only on human mIgE on B cells and not on free, soluble IgE, have been prepared and are under clinical development for the treatment of allergy and asthma.[29][30] An anti-M1’ humanized antibody, quilizumab, is in phase IIb clinical trial.[31][32]
In 2002, researchers at The Randall Division of Cell and Molecular Biophysics determined the structure of IgE.[33] Understanding of this structure (which is atypical of other isotypes in that it is highly bent and asymmetric) and of the interaction of IgE with receptor FcεRI will enable development of a new generation of allergy drugs that seek to interfere with the IgE-receptor interaction. It may be possible to design treatments cheaper than monoclonal antibodies (for instance, small molecule drugs) that use a similar approach to inhibit binding of IgE to its receptor.
References
- ↑ "Antibody structure".
- ↑ Erb KJ (2007). "Helminths, allergic disorders and IgE-mediated immune responses: where do we stand?". Eur. J. Immunol. 37 (5): 1170–3. doi:10.1002/eji.200737314. PMID 17447233.
- ↑ Fitzsimmons CM, McBeath R, Joseph S, Jones FM, Walter K, Hoffmann KF et al. (2007). "Factors affecting human IgE and IgG responses to allergen-like Schistosoma mansoni antigens: Molecular structure and patterns of in vivo exposure". Int. Arch. Allergy Immunol. 142 (1): 40–50. doi:10.1159/000095997. PMID 17019080.
- ↑ Watanabe N, Bruschi F, Korenaga M (2005). "IgE: a question of protective immunity in Trichinella spiralis infection". Trends Parasitol. 21 (4): 175–8. doi:10.1016/j.pt.2005.02.010. PMID 15780839.
- ↑ Pfister K, Turner K, Currie A, Hall E, Jarrett EE (1983). "IgE production in rat fascioliasis". Parasite Immunol. 5 (6): 587–93. doi:10.1111/j.1365-3024.1983.tb00775.x. PMID 6657297.
- ↑ Duarte J, Deshpande P, Guiyedi V, Mécheri S, Fesel C, Cazenave PA et al. (2007). "Total and functional parasite specific IgE responses in Plasmodium falciparum-infected patients exhibiting different clinical status". Malar. J. 6: 1. doi:10.1186/1475-2875-6-1. PMC 1781948. PMID 17204149.
- ↑ Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA et al. (2003). "The biology of IGE and the basis of allergic disease". Annu. Rev. Immunol. 21: 579–628. doi:10.1146/annurev.immunol.21.120601.141103. PMID 12500981.
- ↑ Winter WE, Hardt NS, Fuhrman S (2000). "Immunoglobulin E: importance in parasitic infections and hypersensitivity responses". Arch. Pathol. Lab. Med. 124 (9): 1382–5. doi:10.1043/0003-9985(2000)124<1382:IE>2.0.CO;2 (inactive 2015-01-14). PMID 10975945.
- ↑ Lichtenstein LM, Norman PS, Winkenwerder WL, Osler AG (1966). "In vitro studies of human ragweed allergy: changes in cellular and humoral activity associated with specific desensitization". J. Clin. Invest. 45 (7): 1126–36. doi:10.1172/JCI105419. PMC 292785.
- ↑ Ishizaka K, Ishizaka T, Hornbrook MM (1966). "Physico-chemical properties of human reaginic antibody. IV. Presence of a unique immunoglobulin as a carrier of reaginic activity". J. Immunol. 97 (1): 75–85. PMID 4162440.
- ↑ Creticos PS (2007). "Legends in allergy: Philip S. Norman and Lawrence M. Lichtenstein--the Hopkins experience". J. Allergy Clin. Immunol. 119 (4): 1031–8. doi:10.1016/j.jaci.2007.02.035.
- ↑ Ewart MA, Ozanne BW, Cushley W (May 2002). "The CD23a and CD23b proximal promoters display different sensitivities to exogenous stimuli in B lymphocytes". Genes Immun. 3 (3): 158–64. doi:10.1038/sj.gene.6363848. PMID 12070780.
- ↑ Novak N, Kraft S, Bieber T (December 2001). "IgE receptors". Curr. Opin. Immunol. 13 (6): 721–6. PMID 11677096.
- ↑ Rihet P, Demeure CE, Bourgois A, Prata A, Dessein AJ (1991). "Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels". Eur. J. Immunol. 21 (11): 2679–86. doi:10.1002/eji.1830211106. PMID 1936116.
- ↑ Pritchard DI, Quinnell RJ, Walsh EA (1995). "Immunity in humans to Necator americanus: IgE, parasite weight and fecundity". Parasite Immunol. 17 (2): 71–5. doi:10.1111/j.1365-3024.1995.tb00968.x. PMID 7761110.
- ↑ Turner JD et al. (2005). "Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection". Microbes Infect. 7 (7-8): 990–6. doi:10.1016/j.micinf.2005.03.036. PMID 15961339.
- ↑ Karagiannis SN, Wang Q, East N, Burke F, Riffard S, Bracher MG et al. (2003). "Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells". Eur. J. Immunol. 33 (4): 1030–40. doi:10.1002/eji.200323185. PMID 12672069.
- ↑ Busse W, Buhl R, Fernandez Vidaurre C, Blogg M, Zhu J, Eisner MD et al. (April 2012). "Omalizumab and the risk of malignancy: results from a pooled analysis". J. Allergy Clin. Immunol. 129 (4): 983–9.e6. doi:10.1016/j.jaci.2012.01.033. PMID 22365654.
- ↑ Takhar P, Smurthwaite L, Coker HA, Fear DJ, Banfield GK, Carr VA et al. (2005). "Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis". J. Immunol. 174 (8): 5024–32. doi:10.4049/jimmunol.174.8.5024. PMID 15814733.
- ↑ Permin H, Wiik A (1978). "The prevalence of IgE antinuclear antibodies in rheumatoid arthritis and systemic lupus erythematosus". Acta Pathol Microbiol Scand C 86C (5): 245–9. PMID 309705.
- ↑ Elkayam O, Tamir R, Pick AI, Wysenbeek A (September 1995). "Serum IgE concentrations, disease activity, and atopic disorders in systemic lupus erythematosus". Allergy 50 (1): 94–6. PMID 7741196.
- ↑ Conrad DH, Ford JW, Sturgill JL, Gibb DR (September 2007). "CD23: an overlooked regulator of allergic disease". Curr Allergy Asthma Rep 7 (5): 331–7. doi:10.1007/s11882-007-0050-y. PMID 17697638.
- ↑ Holm J, Willumsen N, Würtzen PA, Christensen LH, Lund K (April 2011). "Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity". J. Allergy Clin. Immunol. 127 (4): 1029–37. doi:10.1016/j.jaci.2011.01.062. PMID 21377718.
- ↑ Cox L, Williams B, Sicherer S, Oppenheimer J, Sher L, Hamilton R et al. (2008). "Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force". Ann. Allergy Asthma Immunol. 101 (6): 580–92. doi:10.1016/S1081-1206(10)60220-7. PMID 19119701.
- ↑ American Academy of Allergy, Asthma, and Immunology. "Five Things Physicians and Patients Should Question" (PDF). Choosing Wisely: an initiative of the ABIM Foundation (American Academy of Allergy, Asthma, and Immunology). Retrieved August 14, 2012
- ↑ Chang TW, Wu PC, Hsu CL, Hung AF (2007). "Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases". Adv. Immunol. Advances in Immunology 93: 63–119. doi:10.1016/S0065-2776(06)93002-8. ISBN 9780123737076. PMID 17383539.
- ↑ "Novartis announces Xolair® approved in EU as first and only licensed therapy for chronic spontaneous urticaria (CSU) patients unresponsive to antihistamines". Novartis. 2014-03-06. Retrieved 2014-12-04.
- ↑ "Novartis announces US FDA approval of Xolair® for chronic idiopathic urticaria (CIU)". Novartis. 2014-03-21. Retrieved 2014-12-04.
- ↑ Chen JB, Wu PC, Hung AF, Chu CY, Tsai TF, Yu HM et al. (February 2010). "Unique epitopes on C epsilon mX in IgE-B cell receptors are potentially applicable for targeting IgE-committed B cells". J. Immunol. 184 (4): 1748–56. doi:10.4049/jimmunol.0902437. PMID 20083663.
- ↑ Brightbill HD, Jeet S, Lin Z, Yan D, Zhou M, Tan M et al. (June 2010). "Antibodies specific for a segment of human membrane IgE deplete IgE-producing B cells in humanized mice". J. Clin. Invest. 120 (6): 2218–29. doi:10.1172/JCI40141. PMC 2877936. PMID 20458139.
- ↑ "MEMP1972A". ClinicalTrials.gov. U.S. National Institutes of Health. Retrieved 2014-12-04.
- ↑ Gauvreau GM, Harris JM, Boulet LP, Scheerens H, Fitzgerald JM, Putnam WS et al. (2 July 2014). "Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production". Sci Transl Med 6 (243): 243ra85. doi:10.1126/scitranslmed.3008961. PMID 24990880.
- ↑ Wan T, Beavil RL, Fabiane SM, Beavil AJ, Sohi MK, Keown M et al. (2002). "The crystal structure of IgE Fc reveals an asymmetrically bent conformation". Nat. Immunol. 3 (7): 681–6. doi:10.1038/ni811. PMID 12068291.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||