Iceane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetracyclo[5.3.1.12,604,9]dodecane | |||
| Other names
Wurtzitane | |||
| Identifiers | |||
| 53283-19-5 | |||
| ChemSpider | 26333278 | ||
| |||
| Jmol-3D images | Image | ||
| |||
| Properties | |||
| C12H18 | |||
| Molar mass | 163.56 g/mol | ||
| Structure | |||
| Point group | D3h | ||
| Dipole moment | 0 D | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
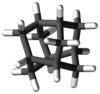
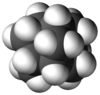
Iceane is a saturated polycyclic hydrocarbon with formula C12H18. It has a cage-like molecular structure, whose carbon skeleton can be viewed as three fused cyclohexane rings in the "boat" conformation; or as two such rings in the "chair" conformation, connected by three parallel bonds.
The name "iceane" was proposed by the chemist Louis Fieser about a decade before the compound was first prepared. He was carrying out studies on the arrangement of water molecules in ice, when it occurred to him that there could exist a stable hydrocarbon with the above structure.
It is also referred to as wurtzitane,[1] due to its similarity to the wurtzite crystal structure;[2] however, the name "iceane" has precedence.
See also
References
- ↑ Tobler, Hans; Klaus, Rolf Otto; Ganter, Camille (1975). "Wurtzitan (Tetracyclo\5.3.1.12,6.04,9]dodecan)". Helvetica Chimica Acta 58 (5): 1455. doi:10.1002/hlca.19750580522.
- ↑ Hamon, DPG; Taylor, GF (1976). "A synthesis of tetracyclo\5,3,1,12,6,04,9]dodecane (iceane)". Australian Journal of Chemistry 29 (8): 1721. doi:10.1071/CH9761721.
External links
- Royal Society of Chemistry Journal
- Symmetry Through the Eyes of a Chemist, Magdolna Hargittai
- A Basis for Synthesis Design, Tse-Lok Ho
- Structures and Energies of Polycyclic Hydrocarbons, Joan E. Shields