Hypusine
 | |
| Names | |
|---|---|
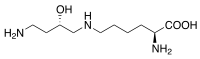
| IUPAC name
2-Amino-6-[(4-amino-2-hydroxybutyl)amino]hexanoic acid[1] | |
| Other names
N6-(4-Amino-2-hydroxybutyl)lysine | |
| Identifiers | |
| 34994-11-1 (2S)-2-Amino, -6-{[(2R)-2-hydroxybutyl]amino} | |
| ChEBI | CHEBI:21858 |
| ChemSpider | 10624726 16740599 (2S)-2-Amino 9097677 (2S)-2-Amino, -6-{[(2R)-2-hydroxybutyl]amino} 58862 (2S)-2-Amino, -6-{[(2S)-2-hydroxybutyl]amino} |
| |
| Jmol-3D images | Image Image |
| MeSH | hypusine |
| PubChem | 21878228 15930878 (2S)-2-Amino 10922432 (2S)-2-Amino, -6-{[(2R)-2-hydroxybutyl]amino} 65396 (2S)-2-Amino, -6-{[(2S)-2-hydroxybutyl]amino} |
| |
| Properties | |
| Molecular formula |
C10H23N3O3 |
| Molar mass | 233.31 g·mol−1 |
| Related compounds | |
| Related alkanoic acids |
|
| Related compounds |
Palmitoylethanolamide |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Hypusine is an unusual amino acid found in all eukaryotes and in some archaea, but not in bacteria. The only known protein containing hypusine is eukaryotic translation initiation factor 5A (eIF5A) and a similar protein found in archaebacteria.[2] In humans, two isoforms of eIF-5A have been described: eIF5A-1 and eIF5A-2. They are encoded by two different genes EIF5A and EIF5A2. The protein is involved in protein biosynthesis and promotes the formation of the first peptide bond. The region surrounding the hypusine residue is highly conserved and is essential to the function of eIF5A.[3] Thus, hypusine and eIF-5A appear to be vital for the viability and proliferation of eukaryotic cells.
Hypusine is formed in eIF-5A by post-translational modification of one of the lysyl residues. There are two reactions and two enzymes involved:
- 1. Deoxyhypusine synthase catalyzes the cleavage of the polyamine spermidine and transfer of its 4-aminobutyl moiety to the ε-amino group of one specific lysine residue of the eIF-5A precursor to form deoxyhypusine and 1,3-diaminopropane.
- 2. Deoxyhypusine hydroxylase mediates the formation of hypusine by addition of a hydroxyl group to the deoxyhypusine residue.
An excess of hypusine was found in the urine of children and patients with familial hyperlysinemia.
Hypusine was first isolated from bovine brain by Japanese scientists Shiba et al. in 1971.[4] The name hypusine indicates that the molecule comprises moieties of hydroxyputrescine and lysine.
References
- ↑ "34994-11-1 - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 5 December 2007. Identification. Retrieved 29 April 2012.
- ↑ Park MH (2006). "The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A)". Journal of Biochemistry 139 (2): 161–169. doi:10.1093/jb/mvj034. PMC 2494880. PMID 16452303.
- ↑ Cano VS, Jeon GA, Johansson HE, Henderson CA, Park JH, Valentini SR, Hershey JW, Park MH (2008). "Mutational analyses of human eIF5A-1 -- Identification of amino acid residues critical for eIF5A activity and hypusine modification". FEBS Journal 275 (1): 44–58. doi:10.1111/j.1742-4658.2007.06172.x. PMC 2536608. PMID 18067580.
- ↑ Shiba T, Mizote H, Kaneko T, Nakajima T, Kakimoto Y, Isamu S (1971). "Hypusine, a new amino acid occurring in bovine brain. Isolation and structural determination". Biochimica et Biophysica Acta 244 (3): 523–531. doi:10.1016/0304-4165(71)90069-9. PMID 4334286.