Hydnellum
| Hydnellum | |
|---|---|
 | |
| The bleeding tooth fungus – Hydnellum peckii | |
| Scientific classification | |
| Kingdom: | Fungi |
| Division: | Basidiomycota |
| Class: | Agaricomycetes |
| Order: | Thelephorales |
| Family: | Bankeraceae |
| Genus: | Hydnellum P.Karst. (1879) |
| Type species | |
| Hydnum suaveolens Scop. (1772) | |
| Synonyms[1] | |
| |
Hydnellum is a genus of tooth fungi of the family Bankeraceae in the order Thelephorales. Widely distributed in the Northern Hemisphere, the genus contains around 40 species. The fruitbodies of its members grow by slowly enveloping nearby bits of grass and vegetation. There is great variability in the form of Hydnellum fruitbodies, which are greatly influenced by environmental conditions such as rainfall and humidity, drying winds, and temperature. They are too tough and woody to eat comfortably. Several species have become the focus of increasing conservation concern following widespread declines in abundance.
Hydnellum species produce pigments that have been used to dye textiles. Several chemical compounds—some with unique biological activity—have been isolated and identified from the genus.
One of the better-known species is the unusual pinkish-white Hydnellum peckii, also known as "strawberries and cream" or as the "bleeding tooth fungus" due to the red droplets that appear on the pinkish or whitish fruitbodies. Another species, H. suaveolens, has a strong odor of anise or peppermint.
Taxonomy
Hydnellum was circumscribed by Finnish mycologist Petter Adolf Karsten in 1879 with what was then known as Hydnum suaveolens as the type species.[2] Before then, fungi with spines (hydnoid fungi) had been grouped in Hydnum by Elias Fries in his 1821 work Systema mycologicum.[3] Karsten defined Hydnellum as having fruitbodies with a corky or leathery, tough cap, and a centrally attached stipe.[2] Synonyms of Hydnellum include Calodon (Karsten, 1881[4]), and Phaeodon (Joseph Schröter, 1888[5]).[1]
Hydnellum is classified in the family Bankeraceae, which was circumscribed by Marinus Anton Donk in 1961. The genus was not in Donk's original family concept, which included only Bankera and Phellodon, genera whose species made hyaline (translucent), and ornamented spores. Donk left Hydnellum in the tribe Hydnelleae of the family Thelephoraceae, along with Sarcodon and Hydnodon.[6] In 1981,[7] however, Walter Jülich emended Donk's concept of the Bankeraceae, adding hydnoid genera that produced brown, lobed spores—Hydnellum and Sarcodon.[8]
The name comes the Greek hydnum meaning spongy plant or fungus.[9] The British Mycological Society, in their recommended list of common names for fungi in the United Kingdom, name Hydnellum fungi in the form "descriptor word" plus "tooth", such as "gold tooth" (H. auratile), "zoned tooth" (H. concrescens), and "velvet tooth" (H. spongiosipes).[10]
Description
Hydnellum fruitbodies have caps and stipes, often with indeterminant growth forms, that may grow in spurts and decay over several weeks.[11] Neighboring fruitbodies can coalesce, forming intricately intertwined caps and partially fused stipes.[12] The flesh has a zoned appearance and is fibrous when fresh, but becomes hard and woody when dry.[11] Zones in the flesh reflect differences in growth during periods of low daytime and high nighttime humidity, and give a fairly accurate record of daily growth.[13] The spines are crowded closely together and typically decurrent (extending down the length of the stipe). They may be a variety of colors, such as white to yellow, olive green, shades of orange, light brown, or dark brown in age.[11]
Spores of Hydnellum are almost spherical to oblong and tuberculate, and are brown in mass.[11] The basidia (spore-bearing cells) are narrowly club-shaped and usually four-spored; there are no cystidia in Hydnellum.[14] Three types of hyphae are found in the flesh of Hydnellum: generative hyphae (thin-walled, not inflated); skeletal hyphae (thick-walled and narrow); and thin-walled gloeoplerous-like hyphae, which stain with methyl blue.[15]

In conditions of high humidity, several species can form striking colored drops on the actively growing caps: red drops in H. peckii, H. diabolus, H. ferrugineum, and H. cruentum, yellow drops in H. caeruleum, and coffee-colored drops in H. mirabile.[16] The common names of H. peckii reflect its appearance: "strawberries and cream" and "bleeding tooth fungus".[17] Some Hydnellum species have a mealy odor (e.g. H. mirabile and H. pineticola) similar to freshly ground flour. H. zonatum smells like melilot,[18] while H. suaveolens has an sweet odor resembling anise or peppermint. All are too tough and woody to be edible, and many have an acrid taste anyway.[17]
Differences between Hydnellum species tend to be more distinguishable in younger specimens. Fruitbody development is greatly influenced by environmental factors such as levels of rainfall, drying winds, and temperature.[19] The blue tooth (H. caeruleum), for example, develops a deeper blue color when it grows during cooler autumn weather.[19] Optimal growth occurs during periods of frequent light rains and high humidity; if the habitat dries out, growth will stop, but may resume after further precipitation. This intermittent growth affects the fruitbodies of different species to variable extents, leading to large variations in form, surface texture, and color.[19] The morphological variability of fruitbodies and the dependence of their appearance upon environmental conditions has made Hydnellum a difficult group to study. Canadian mycologist Kenneth Harrison, who described several new species from North America, noted "[t]he remarkable longevity of individual sporophores of many species and the changes in appearance that occur during the long period of their development have confused all workers studying this group."[20] For example, H. aurantiacum, initially white, becomes in turn shades of orange, rusty-brown, and brownish-black. Its fruitbody initially has a turbinate (cushion-like) shape with a lumpy surface, later becoming flattened to funnel-shaped with a smooth to corrugated surface texture.[12]


The caps form from the top of the short stipe by the growth and expansion of a blunt margin and later as a thickening of the upper surface.[12] Spines start to form when the cap hangs over the stipe slightly. They are white in many species, but become brown in maturity as the brown-colored spores accumulate on the surface.[19]
Habitat and distribution
Hydnellum fungi are mycorrhizal, and are usually found in coniferous and mixed woods.[14] Favored tree hosts include members of the Fagaceae and the Pinaceae.[21] The genus is widely distributed in the Northern Hemisphere, particularly Europe and North America, but some species are found in the tropical Asia.[14] Harrison identified a dozen new species from North America in the 1960s.[22][23][13] Rudolph Arnold Maas Geesteranus recognized European 16 species in his 1975 treatment of the genus.[24]
Some Hydnellum species, including H. ferrugineum and H. scleropodium, form a tough mat of mycelia in the humus and upper soil of pine forests. This mycelial mat grows larger with old trees, and can cover an area of several square meters. These areas generally lack dwarf shrubs and promote the vigorous growth of mosses; reindeer lichens often occur in the center of large mats. The presence of the fungus changes the nature of the soil, resulting in a thinner humus layer, decreased groundwater penetration, decreased soil pH, and increases in the level of root respiration as well as the quantity of roots. The fungus also decreases the organic carbon and nitrogen concentrations. Soil with the mycelium becomes more podzolized than the surrounding soil.[25][26]
Conservation

Some Hydnellum species have been shown to be in decline in Europe, including the Czech Republic,[27] the Netherlands,[28] Norway,[29] and Scotland.[30] In the United Kingdom, several are listed in the biodiversity action plan for stiped hydnoid fungi: H. aurantiacum is classified as critically endangered; H. caeruleum, H. ferrugineum are listed as endangered, while H. concrescens, H. spongiosipes, H. peckii, and H. scrobiculatum are considered vulnerable.[31] H. ferrugineum and H. peckii are sensitive to the increased nitrogen deposition resulting from clear-cutting, a forestry practice used in some areas of Europe.[32]
Conservation efforts for Hydnellum are hindered by the fact that some species are difficult to discriminate in the field, making it hard to determine an appropriate conservation status.[33] Techniques based on species-specific PCR primers and DNA extraction from soil have been developed to detect the mycelia of various Hydnellum species without having to rely on the presence of fruitbodies, which may help conservation efforts as well as improve understanding of below-ground ecology.[34] Similar techniques have been used to show that, in the case of H. aurantiacum and H. caeruleum, the fungus can persist below the ground for at least four years without producing fruitbodies.[35]
Bioactive compounds
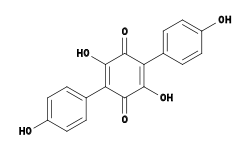
Several chemical compounds—some with unique biological activity—have been isolated and identified from Hydnellum species. For example, H. peckii contains atromentin, a pigment with anticoagulant properties similar to heparin.[36] Atromentin also possesses antibacterial activity, inhibiting the enzyme enoyl-acyl carrier protein reductase (essential for the biosynthesis of fatty acids) in the bacteria Streptococcus pneumoniae.[37]
Some species are used as dyes. H. caeruleum, used in North America and Scandinavia to dye silk and wool, produces a range of colors including tan, blue, and forest-green, depending on the mordant that is used. H. peckii produces gray, brown, and olive colors.[38] Hydnuferrugin and hydnuferruginin are pigments responsible for the coloration of H. ferrugineum and H. zonatum.[39] Geogenin is a yellow pigment found in H. geogenium.[40]
Thelephoric acid is present in several Hydnellum species.[41] This compound, derived metabolically from the shikimic acid pathway, inhibits the enzyme prolyl endopeptidase, which is involved in deteriorating certain neuropeptides that are believed to contribute to memory and learning.[42] Hydnellum caeruleum and H. concrescens have several para-terphenyl derivatives named thelephantins,[43] some of which can inhibit the enzyme alpha-glucosidase.[44] The compounds hydnellins A and B are terphenyls found in H. suaveolens and H. geogerirum.[45] The chemicals responsible for the fragrant anise-like aroma of H. suaveolens have been identified as coumarin and para-anisaldehyde.[46]
Species
Karsten's original 1879 circumscription of Hydnellum contained 19 species.[2] Joost Stalpers included 34 Hydnellum species in his 1993 monograph on the Thelephorales.[47] The tenth edition of the Dictionary of the Fungi (2008) indicated 38 species in the genus.[48] As of January 2015, Index Fungorum lists 39 species of Hydnellum.[49]



- Hydnellum aurantiacum (Batsch) P.Karst. (1879) – Asia, Europe, North America[14]
- Hydnellum auratile (Britzelm.) Maas Geest. (1959) – Europe, North America[50]
- Hydnellum caeruleum (Hornem.) P.Karst. (1879) – Asia, Europe, North America[14]
- Hydnellum chrysinum K.A.Harrison (1964) – North America[23]
- Hydnellum coalitum Maas Geest. (1975) – Europe[24]
- Hydnellum compactum (Pers.) P.Karst. (1879) – Europe[51]
- Hydnellum complicatum Banker (1906) – North America[52]
- Hydnellum concrescens (Pers.) Banker (1906) – Asia, Europe, North America[14]
- Hydnellum conigenum (Peck) Banker (1906) – North America[53]
- Hydnellum cristatum (Bres.) Stalpers (1993) – Europe, North America
- Hydnellum cruentum K.A.Harrison (1961) – Nova Scotia, Canada[54]
- Hydnellum crustulinum Maas Geest. (1971) – Punjab, India[51]
- Hydnellum cumulatum K.A.Harrison (1964) – Europe,[55] North America[23]
- Hydnellum cyanodon K.A.Harrison (1964) – North America[23]
- Hydnellum cyanopodium K.A.Harrison (1964) – North America[23]
- Hydnellum earlianum Banker (1906) – North America[52]
- Hydnellum ferrugineum (Fr.) P.Karst. (1879) – North Africa, Asia, Europe, North America[14]
- Hydnellum floriforme (Schaeff.) Banker (1906) – North America[52]
- Hydnellum fraudulentum Maas Geest. (1971) – Australia[51]
- Hydnellum frondosum K.A.Harrison (1961) – Nova Scotia, Canada[56]
- Hydnellum geogenium (Fr.) Banker (1913) – Europe, North America[57]
- Hydnellum gracilipes (P.Karst.) P.Karst. (1879) – Europe[58]
- Hydnellum longidentatum Coker (1939) – United States[59]
- Hydnellum mirabile (Fr.) P.Karst. (1879) – Europe, North America[60]
- Hydnellum multiceps K.A.Harrison (1961) – Nova Scotia, Canada[56]
- Hydnellum nigellum K.A.Harrison (1964) – North America[23]
- Hydnellum papuanum Maas Geest. (1971) – Papua New Guinea[51]
- Hydnellum peckii Banker (1912) – Europe, North America[14]
- Hydnellum regium K.A.Harrison (1964) – North America[23]
- Hydnellum rickeri Banker (1913) – North America[61]
- Hydnellum scleropodium K.A.Harrison (1964) – North America[23]
- Hydnellum scrobiculatum (Fr.) P.Karst. (1879) – Asia, Europe, North America[14]
- Hydnellum septentrionale K.A.Harrison (1964) – North America[23]
- Hydnellum singeri Maas Geest. (1969) – Colombia[62]
- Hydnellum spongiosipes (Peck) Pouzar (1960) – Europe, North America[14]
- Hydnellum staurastrum Maas Geest. (1971) – Malaysia[51]
- Hydnellum suaveolens (Scop.) P.Karst. (1879) – Asia, Europe, North America[63]
- Hydnellum subzonatum K.A.Harrison (1961) – Nova Scotia, Canada[54]
- Hydnellum tardum Maas Geest. (1975) – Europe[24]
References
- ↑ 1.0 1.1 "Hydnellum P. Karst. 1879". MycoBank. International Mycological Association. Retrieved 2012-03-25.
- ↑ 2.0 2.1 2.2 Karsten PA. (1879). "Symbolae ad mycologiam Fennicam. VI". Meddelanden af Societas pro Fauna et Flora Fennica 5: 5–46 (see p. 41).
- ↑ Harrison (1961), p. 16.
- ↑ Karsten PA. (1881). "Enumeratio Hydnearum Fr. Fennicarum, systemate novo dispositarum". Revue Mycologique Toulouse (in Latin) 3 (9): 19–21.
- ↑ Schröter J. (1888). Kryptogamen-Flora von Schlesien. 3–1(4). Lehre, Germany: Cramer. p. 458.
- ↑ Donk MA. (1961). "Four new families of Hymenomycetes". Persoonia 1 (4): 405–7.
- ↑ Jülich W. (1981). Higher taxa of Basidiomycetes. Bibliotheca Mycologica 85. Vaduz, Liechtenstein: J. Cramer. ISBN 978-3-7682-1324-0.
- ↑ Stalpers (1993), p. 22.
- ↑ Gamiet S, Berch SM, Kroeger P, Roberts C, Winder R, MacKinnon A. "Welcome to BC NTFP Mushrooms". BC NTFP mushrooms. Southern Interior Forest Extension and Research Partnership. Retrieved 2010-02-12.
- ↑ "Recommended English Names for Fungi in the UK" (PDF). British Mycological Society.
- ↑ 11.0 11.1 11.2 11.3 Baird RE, Khan SR. (1986). "The stipitate Hydnums (Thelephoraceae) of Florida USA". Brittonia 38 (2): 171–84. doi:10.2307/2807273. JSTOR 2807273.
- ↑ 12.0 12.1 12.2 Harrison (1961), p. 13.
- ↑ 13.0 13.1 Harrison KA. (1968). "Studies on the Hydnums of Michigan I. Genera Phellodon, Bankera, Hydnellum". Michigan Botanist 7: 212–64.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 14.8 14.9 Pegler DN, Roberts PJ, Spooner BM. (1997). British Chanterelles and Tooth Fungi. Kew, UK: Royal Botanic Gardens. pp. 74–88. ISBN 978-1-900347-15-0.
- ↑ Stalpers (1997), p. 9.
- ↑ Harrison (1961), p. 15.
- ↑ 17.0 17.1 Arora D. (1986). Mushrooms Demystified. Berkeley, California: Ten Speed Press. pp. 623–27. ISBN 0-89815-169-4.
- ↑ Rumack BH, Spoerke DG. (1994). Handbook of Mushroom Poisoning: Diagnosis and Treatment. CRC Press. p. 413. ISBN 978-0-8493-0194-0.
- ↑ 19.0 19.1 19.2 19.3 Harrison (1961), p. 14.
- ↑ Harrison (1961), p. 12.
- ↑ Stalpers (1997), p. 34.
- ↑ Harrison (1961).
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 23.8 Harrison KA. (1964). "New or little known North American stipitate Hydnums". Canadian Journal of Botany 42 (9): 1205–33. doi:10.1139/b64-116.
- ↑ 24.0 24.1 24.2 Maas Geesteranus RA. (1975). "Die terrestrischen Stachelpilze Europas". Verhandelingen Koninklijke Nederlandse Akademie van Wetenschappen Afdeling Natuurkunde (in German) 64 (2): 1–127 (see pp. 95–98).
- ↑ Hintikka V, Näykki O. (1967). "Tutkimuksia ruosteorakkaan, Hydnellum ferrugineum, vaikutuksesta metsämaaperään ja-kasvillisuuteen [Notes on the effects of the fungus Hydnellum ferrugineum (Fr.) Karst. on forest soil and vegetation]". Communicationes Instituti Forestalis Fenniae 62 (2): 1–23.
- ↑ Fisher RF. (1972). "Spodosol development and nutrient distribution under Hydnaceae fungal mats". Proceedings of the Soil Science Society of America 36 (3): 492–5. doi:10.2136/sssaj1972.03615995003600030035x.
- ↑ Hrouda P. (1999). "Hydnaceous fungi of the Czech Republic and Slovakia". Czech Mycology 51: 99–155.
- ↑ Arnolds E. (1989). "Former and present distribution of stipitate hydnaceous fungi (Basidiomycetes) in the Netherlands". Nova Hedwigia 68: 107–42.
- ↑ Gulden G, Hanssen EW. (1992). "Distribution and ecology of stipitate hydnaceous fungi in Norway, with special reference to the question of decline". Sommerfeltia 13: 1–58.
- ↑ Newton AC, Holden E, Davy LM, Ward SD, Fleming LV, Watling R. (2002). "Status and distribution of stipitate hydnoid fungi in Scottish coniferous forests". Biological Conservation 107 (2): 181–92. doi:10.1016/S0006-3207(02)00060-5.
- ↑ Brodge PD, Panchal G. (2004). Number 557. Population diversity and speciation in Hydnellum and Phellodon species (PDF) (Report). English Nature Research Reports. English Nature. ISSN 0967-876X.
- ↑ Arnolds E. (2010). "The fate of hydnoid fungi in The Netherlands and Northwestern Europe". Fungal Ecology 3 (2): 81–88. doi:10.1016/j.funeco.2009.05.005.
- ↑ Parfitt D, Ainsworth AM, Simpson D, Rogers HJ, Boddy L. (2007). "Molecular and morphological discrimination of stipitate hydnoids in the genera Hydnellum and Phellodon". Mycological Research 111 (7): 761–77. doi:10.1016/j.mycres.2007.05.003. PMID 17681224.
- ↑ van der Linde S, Alexander I, Anderson IC. (2008). "A PCR-based method for detecting the mycelia of stipitate hydnoid fungi in soil". Journal of Microbiological Methods 75 (1): 40–46. doi:10.1016/j.mimet.2008.04.010. PMID 18586344.
- ↑ van der Linde S, Holden E, Parkin PI, Alexander IJ, Anderson IC. (2012). "Now you see it, now you don’t: The challenge of detecting, monitoring and conserving ectomycorrhizal fungi". Fungal Ecology 5 (5): 633–40. doi:10.1016/j.funeco.2012.04.002.
- ↑ Khanna JM, Malone MH, Euler KL, Brady LR. (1965). "Atromentin – anticoagulant from Hydnellum diabolus". Journal of Pharmaceutical Science 54 (7): 1016–20. doi:10.1002/jps.2600540714. PMID 5862512.
- ↑ Zheng CJ, Sohn MJ, Kim WG. (2006). "Atromentin and leucomelone, the first inhibitors specific to enoyl-ACP reductase (FabK) of Streptococcus pneumoniae". Journal of Antibiotics 59 (12): 808–12. doi:10.1038/ja.2006.108. PMID 17323650.
- ↑ Roberts P, Evans S. (2011). The Book of Fungi. Chicago, Illinois: University of Chicago Press. pp. 469–70. ISBN 978-0-226-72117-0.
- ↑ Gripenberg J. (1981). "Fungus pigments. XXIX. The pigments of Hydnellum ferrugineum (Fr.) Karsten and H. zonatum (Batsch) Karsten". Acta Chemica Scandinavica B 35: 513–9.
- ↑ Steglich W, Jendrny B. (1979). "Geogenin, ein neuartiger Pyronfarbstoff aus Hydnellum geogenium" [Geogenin, a new kind of pyrone pigment from Hydnellum geogenium]. Beihefte Zur Sydowia Annales Mycologici Ser II (in German): 378–80.
- ↑ Stalpers (1997), p. 7.
- ↑ Kwak JY, Rhee IK, Lee KB, Hwang JS, Yoo ID, Song KS. (1999). "Thelephoric acid and kynapcin-9 in mushroom Polyozellus multiplex inhibit prolyl endopeptidase in vitro". Journal of Microbiology and Biotechnology 9 (6): 798–803.
- ↑ Quang DN, Hashimoto T, Hitaka Y, Tanaka M, Nukada M, Yamamoto I, Asakawa Y. (2004). "Thelephantins I-N; p-terphenyl derivatives from the inedible mushroom Hydnellum caeruleum". Phytochemistry 65 (8): 1179–84. doi:10.1016/j.phytochem.2004.02.018. PMID 15110701.
- ↑ Wang SM, Han JJ, Ma K, Jin T, Bao L, Pei YF, Liu HW. (2014). "New α-glucosidase inhibitors with p-terphenyl skeleton from the mushroom Hydnellum concrescens". Fitoterapia 98: 149–55. doi:10.1016/j.fitote.2014.07.019. PMID 25088970.
- ↑ Hashimoto T, Quang DN, Kuratsune M, Asakawa Y. (2006). "Hydnellins A and B, nitrogen-containing terphenyls from the mushrooms Hydnellum suaveolens and Hydnellum geogerirum". Chemical and Pharmaceutical Bulletin 54 (6): 912–4. doi:10.1248/cpb.54.912.

- ↑ Wood WF, DeShazer DA, Largent DL. (1988). "The identify and metabolic fate of volatiles responsible for the odor of Hydnellum suaveolens". Mycologia 82 (2): 252–5.
- ↑ Stalpers (1993).
- ↑ Kirk PM, Cannon PF, Minter DW, Stalpers JA. (2008). Dictionary of the Fungi (10th ed.). Wallingford, UK: CAB International. p. 324. ISBN 978-0-85199-826-8.
- ↑ Kirk PM. "Species Fungorum (version 22nd December 2014). In: Species 2000 & ITIS Catalogue of Life". Retrieved 2015-01-03.
- ↑ Maas Geesteranus RA. (1959). "Sur un Hydnellum méconnu". Persoonia (in French) 1 (1): 111–4.
- ↑ 51.0 51.1 51.2 51.3 51.4 Maas Geesteranus RA. (1971). "Hydnaceous fungi of the eastern old world". Verhandelingen Koninklijke Nederlandse Akademie van Wetenschappen Afdeling Natuurkunde 60 (3): 1–176 (see pp. 91–106).
- ↑ 52.0 52.1 52.2 Banker HJ. (1906). "A contribution to a revision of the North American Hydnaceae". Memoirs of the Torrey Botanical Club 12: 99–194 (see p. 161).
- ↑ McKnight KH. (1998). A Field Guide to Mushrooms: North America. Houghton Mifflin Harcourt. p. 94. ISBN 978-0-395-91090-0.
- ↑ 54.0 54.1 Harrison (1961), p. 37.
- ↑ Ainsworth M. (2011). "Hydnellum cumulatum and H. gracilipes: two overlooked Scottish hydnoids new to Britain". Field Mycology 12 (4): 139–43. doi:10.1016/j.fldmyc.2011.09.011.
- ↑ 56.0 56.1 Harrison (1961), p. 45.
- ↑ Phillips R. "Hydnellum geogenium". RogersMushrooms. Retrieved 2015-01-06.
- ↑ Kõljalg U, Renvall P. "Hydnellum gracilipes – a link between stipitate and resupinate Hymenomycetes". Karstenia 40 (1–2): 71–77.
- ↑ Coker WC. (1939). "New or noteworthy Basidiomycetes". Journal of the Elisha Mitchell Scientific Society 55: 373–86.
- ↑ Phillips R. "Hydnellum mirabile". RogersMushrooms. Retrieved 2015-01-06.
- ↑ Banker HJ. (1913). "Type studies in the Hydnaceae – V. The genus Hydnellum". Mycologia 5 (4): 194–205. doi:10.2307/3753385.
- ↑ Maas Geesteranus RA. (1969). "Notes on Hydnums, VIII". Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen Section C 72: 213–21.
- ↑ Phillips R. "Hydnellum suaveolens". RogersMushrooms. Retrieved 2015-01-06.
Cited works
- Harrison KA. (1961). The Stipitate Hydnums of Nova Scotia. Publications of the Department of Agriculture Canada (Report) 1099 (Ottawa, Canada: Research Branch, Canada Department of Agriculture): 1–60.

- Stalpers JA. (1993). "The Aphyllophoraceous fungi I. Keys to the species of the Thelephorales". Studies in Mycology 35: 1–168.
External links
| Wikimedia Commons has media related to Hydnellum. |