Human vestigiality

In the context of human evolution, human vestigiality involves those traits (such as organs or behaviors) occurring in humans that have lost all or most of their original function through evolution. Although structures called vestigial often appear functionless, a vestigial structure may retain lesser functions or develop minor new ones. In some cases, structures once identified as vestigial simply had an unrecognized function.
The examples of human vestigiality are numerous, including the anatomical (such as the human appendix, tailbone, wisdom teeth, and inside corner of the eye), the behavioral (goose bumps and palmar grasp reflex), sensory (decreased olfaction), and molecular (noncoding DNA). Many human characteristics are also vestigial in other primates and related animals.
History
In 1893, Robert Wiedersheim published a book on human anatomy and its relevance to man's evolutionary history. This book contained a list of 86 human organs that he considered vestigial, or as Wiedersheim himself explained: "Organs having become wholly or in part functionless, some appearing in the Embryo alone, others present during Life constantly or inconstantly. For the greater part Organs which may be rightly termed Vestigial."[1] In the 1895 edition his wording was subtly, but significantly, different: "By such organs are meant those which were formerly of greater physiological significance than at present."[2] His list of supposedly vestigial organs included many of the examples on this page as well as others then mistakenly believed to be purely vestigial, such as the pineal gland, the thymus gland, and the pituitary gland. Some of these organs that had lost their obvious, original functions later turned out to have retained functions that had gone unrecognized before the discovery of hormones or many of the functions and tissues of the immune system.[3][4] Examples included:
- the role of the pineal in the regulation of the circadian rhythm (neither the function nor even the existence of melatonin was yet known);
- discovery of the role of the thymus in the immune system lay many decades in the future; it remained a mystery organ until after the mid-20th century;
- the pituitary and hypothalamus with their many and varied hormones were far from understood, let alone the complexity of their interrelationships.
Historically there was a trend not only to dismiss the vermiform appendix as being uselessly vestigial, but an anatomical hazard, a liability to dangerous inflammation. As late as the mid 20th century many reputable authorities conceded it no beneficial function.[5] This was a view supported, or perhaps inspired, by Darwin himself in the 1874 edition of his book The Descent of Man, and Selection in Relation to Sex. The organ's patent liability to appendicitis and its poorly understood role left the appendix open to blame for a number of possibly unrelated conditions. For example, in 1916 a surgeon claimed that removal of the appendix had cured several cases of trifacial neuralgia and other nerve pain about the head and face, even though he stated that the evidence for appendicitis in those patients was inconclusive.[6] The discovery of hormones and hormonal principles, notably by Bayliss and Starling argued against these views, but in the early twentieth century there remained a great deal of fundamental research to be done on the functions of large parts of the digestive tract. In 1916 an author found it necessary to argue against the idea that the colon had no important function and that "...the ultimate disappearance of the appendix is a coordinate action and not necessarily associated with such frequent inflammations as we are witnessing in the human..."[7]
There had been a long history of doubt about such dismissive views. Around 1920 the prominent surgeon Kenelm Hutchinson Digby documented previous observations, going back more than thirty years, that suggested lymphatic tissues, such as the tonsils and appendix, may have substantial immunological functions.
Anatomical
Appendix
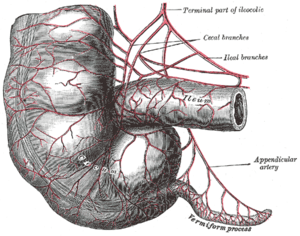
In modern humans, the vermiform appendix is a vestige of a redundant organ that in ancestral species had digestive functions, much as it still does in extant species in which intestinal flora hydrolyze cellulose and similar indigestible plant materials.[8] Some herbivorous animals, such as rabbits, have a terminal vermiform appendix and cecum that apparently bear patches of tissue with immune functions and may also be important in maintaining the composition of intestinal flora. It does not however seem to have much digestive function, if any, and is not present in all herbivores, even those with large caeca.[9] As shown in the accompanying pictures however, the human appendix typically is about comparable to that of the rabbit's in size, though the caecum is reduced to a single bulge where the ileum empties into the colon.[5] Some carnivorous animals may have appendices too, but seldom have more than vestigial caeca.[10] In line with the possibility of vestigial organs developing new functions, some research suggests that the appendix may guard against the loss of symbiotic bacteria that aid in digestion, though that is unlikely to be a novel function, given the presence of vermiform appendices in many herbivores.[11][12] Intestinal bacterial populations entrenched in the appendix may support quick re-establishment of the flora of the large intestine after an illness, poisoning, or antibiotic treatment depletes or otherwise causes harmful changes to the bacterial population of the colon.[13] A 2013 study, however, refutes the idea of an inverse relationship between cecum size and appendix size and presence. It is widely present in euarchontoglires (a superorder of mammals that includes rodents and primates) and has also evolved independently in the diprotodont marsupials, monotremes, and is highly diverse in size and shape which could suggest it is not vestigial.[14]
Coccyx
The coccyx, or tailbone, is the remnant of a lost tail. All mammals have a tail at some point in their development; in humans, it is present for a period of 4 weeks, during stages 14 to 22 of human embryogenesis.[15] This tail is most prominent in human embryos 31–35 days old.[16] The tailbone, located at the end of the spine, has lost its original function in assisting balance and mobility, though it still serves some secondary functions, such as being an attachment point for muscles, which explains why it has not degraded further.
In rare cases congenital defect results in a short tail-like structure being present at birth. Twenty-three cases of human babies born with such a structure have been reported in the medical literature since 1884.[17][18] In rare cases such as these, the spine and skull were determined to be entirely normal. The only abnormality was that of a tail approximately twelve centimeters long. These tails were able to be surgically removed, and the individuals have resumed normal lives.[19]
Wisdom teeth
Wisdom teeth are vestigial third molars that human ancestors used to help in grinding down plant tissue. The common postulation is that the skulls of human ancestors had larger jaws with more teeth, which were possibly used to help chew down foliage to compensate for a lack of ability to efficiently digest the cellulose that makes up a plant cell wall. As human diets changed, smaller jaws were naturally selected, yet the third molars, or "wisdom teeth," still commonly develop in human mouths.[20] Currently, wisdom teeth have become useless and even harmful to the extent where surgical procedures are often performed to remove them.
Agenesis of wisdom teeth in human populations ranges from zero in Tasmanian Aboriginals to nearly 100% in indigenous Mexicans.[21] The difference is related to the PAX9 gene (and perhaps other genes).[22]
Vomeronasal organ
In some animals the vomeronasal organ (VNO) is part of a second, completely separate sense of smell, known as the accessory olfactory system. Many studies have been performed to find if there is an actual presence of a VNO in adult human beings. Trotier et al.[23] estimated that around 92% of their subjects that had no septal surgery had at least one intact VNO. Kjaer and Fisher Hansen, on the other hand,[24] stated that the VNO structure disappeared during fetal development as it does for some primates.[25] However, Smith and Bhatnagar (2000)[26] asserted that Kjaer and Fisher Hansen simply missed the structure in older fetuses. Won (2000) found evidence of a VNO in 13 of his 22 cadavers (59.1%) and in 22 of his 78 living patients (28.2%).[27] Given these findings, some scientists have argued that there is a VNO in adult human beings.[28][29] However, most investigators have sought to identify the opening of the vomeronasal organ in humans, rather than identify the tubular epithelial structure itself.[30] Thus it has been argued that such studies, employing macroscopic observational methods, have sometimes missed or even misidentified the vomeronasal organ.[31]
Among studies that use microanatomical methods, there is no reported evidence that human beings have active sensory neurons like those in working vomeronasal systems of other animals.[31][32] Furthermore, there is no evidence to date that suggests there are nerve and axon connections between any existing sensory receptor cells that may be in the adult human VNO and the brain.[33] Likewise, there is no evidence for any accessory olfactory bulb in adult human beings,[31] and the key genes involved in VNO function in other mammals have become pseudogenes in human beings. Therefore, while the presence of a structure in adult human beings is debated, a review of the scientific literature by Tristram Wyatt concluded, "most in the field ... are sceptical about the likelihood of a functional VNO in adult human beings on current evidence."[34]
Ear
The ears of a Macaque monkey and most other monkeys have far more developed muscles than those of humans, and therefore have the capability to move their ears to better hear potential threats.[35] Humans and other primates such as the orangutan and chimpanzee however have ear muscles that are minimally developed and non-functional, yet still large enough to be identifiable.[8] A muscle attached to the ear that cannot move the ear, for whatever reason, can no longer be said to have any biological function. In humans there is variability in these muscles, such that some people are able to move their ears in various directions, and it can be possible for others to gain such movement by repeated trials.[8][36] In such primates the inability to move the ear is compensated mainly by the ability to turn the head on a horizontal plane, an ability which is not common to most monkeys—a function once provided by one structure is now replaced by another.[37]
The outer structure of the ear also shows some vestigial features, such as the node or point on the helix of the ear known as Darwin's tubercle which is found in around 10% of the population.
Eye
The plica semilunaris is a small fold of tissue on the inside corner of the eye. It is the vestigial remnant of the nictitating membrane, an organ that is fully functional in some other species of mammals.[38] Its associated muscles are also vestigial.[8] Only one species of primate, the Calabar Angwantibo, is known to have a functioning nictitating membrane.[39]
The orbitalis muscle is a vestigial or rudimentary nonstriated muscle (smooth muscle) of the eye that crosses from the infraorbital groove and sphenomaxillary fissure and is intimately united with the periosteum of the orbit. It was described by Johannes Peter Müller and is often called Müller's muscle. The muscle forms an important part of the lateral orbital wall in some animals, but in humans it is not known to have any significant function.
Recently, however, evidence has emerged that Müller's muscle may in fact not be vestigial. This thin, filmy covering has been demonstrated to separate the orbit from the temporal, infratemporal and pterygopalatine fossas. During endoscopic surgery, its identification has become essential for anatomical orientation and is believed now to be important in protruding the eye.[40]
Reproductive system
Genitalia
In the internal genitalia of each human gender, there are some residual organs of mesonephric and paramesonephric ducts during embryonic development:
In some texts regarding the vestigiality of some organs in external genitalia, the nipples in men are cited as the vestigial organs of breasts in women, and the clitoris the vestigial organ of the penis. However, it is better to say that these organs are homologous, as they are present in both genders of humans (and primates), not during the passage of animal development. The hirsuties coronae glandis, also known as "pearly penile papules", which are commonly found in the male sexual organ are described as vestigial remnants of penile spines, sensitive features found in the same location in other primates. In species which retain the full expression of penile spines, the spines contribute to sexual pleasure and quicker orgasms.
Hymen
The hymen is a membrane that surrounds or partially covers the external vaginal opening. Some scientists view the function of hymen in young girls as a protective membrane that protects the reproductive system from infection in the embryonic period and protects the fertility of young girls before mating. The existence of hymen in some animals, such as horses, prevents semen from leaving the vagina. Due to similar reproductive system development, many mammals, including chimpanzees, elephants, manatees, whales, and horses, retain hymens.[41][42]
Musculature
A number of muscles in the human body are thought to be vestigial, either by virtue of being greatly reduced in size compared to homologous muscles in other species, by having become principally tendonous, or by being highly variable in their frequency within or between populations.
Head
The Occipitalis Minor is a muscle in the back of the head which normally joins to the auricular muscles of the ear. This muscle is very sporadic in frequency—always present in Malays, in 56% of Africans, 50% of Japanese, 36% of Europeans, and is nonexistent in the Khoikhoi people of southwestern Africa and in Melanesians.[43] Other small muscles in the head associated with the occipital region and the post-auricular muscle complex are often variable in their frequency.[44]
Face
In many non-human mammals the upper lip and sinus area is associated with whiskers or vibrissae which serve a sensory function. In humans these whiskers do not exist but there are still sporadic cases where elements of the associated vibrissal capsular muscles or sinus hair muscles can be found. Based on histological studies of the upper lips of 20 cadavers, Tamatsu et al. found that structures resembling such muscles were present in 35% (7/20) of their specimens.[45]
Arm
The palmaris longus muscle is seen as a small tendon between the flexor carpi radialis and the flexor carpi ulnaris, although it is not always present. The muscle is absent in about 14% of the population, however this varies greatly with ethnicity. One study has shown the prevalence of palmaris longus agenesis in 500 Indian patients to be 17.2% (8% bilateral and 9.2% unilateral).[46] The palmaris is a popular source of tendon material for grafts and this has prompted studies which have shown the absence of the palmaris does not have any appreciable effect on grip strength.[47]
The levator claviculae muscle in the posterior triangle of the neck is a supernumerary muscle present in only 2–3% of all people[48] but nearly always present in most mammalian species, including gibbons and orangutans.[49]
Torso
The pyramidalis muscle of the abdomen is a small and triangular muscle, anterior to the rectus abdominis, and contained in the rectus sheath. It is absent in 20% of humans and when absent the lower end of the rectus then becomes proportionately increased in size. Anatomical studies suggest that the forces generated by the pyramidalis muscles are relatively small.[50]
Leg
The plantaris muscle is composed of a thin muscle belly and a long thin tendon. The muscle belly is approximately 5–10 centimetres (2–4 inches) long, and is absent in 7–10% of the human population. It has some weak functionality in moving the knee and ankle but is generally considered redundant and is often used as a source of tendon for grafts. The long, thin tendon of the plantaris is humorously called "the freshman's nerve," as it is often mistaken for a nerve by first-year medical students.
Breasts
Extra nipples or breasts sometimes appear along the mammary lines of humans, appearing as a remnant to mammalian ancestors who possessed more than two nipples or breasts.
Tongue
Another intriguing example of human vestigiality occurs in the tongue, specifically the chondroglossus muscle. In a morphological study of 100 Japanese cadavers, it was found that 86% of fibers identified were solid and bundled in the appropriate way to facilitate speech and mastication. The other 14% of fibers were short, thin and sparse – nearly useless, and thus clearly of vestigial origin.[51]
Sensory
Although the sense of smell, or olfaction, is essential for other animals in avoiding predators, finding food, and other functions, olfaction is greatly decreased in humans as they have (for the most part) no predators and obtain food mostly by agriculture. There is great variation in olfactory sensitivity from person to person, which is common in vestigial characteristics. It has been observed that native South Americans, native North Americans, and African peoples have a highly developed sense of smell, such that they may be able to identify others in the dark by their odor alone.[8] This does not mean that having any olfactory ability at all is vestigial, for example it may save a person from inhaling toxic fumes. A characteristic may degenerate despite being of some use if there is very little or no selection pressure on the genes associated with it. In other words, having a good sense of smell may be something a person would desire, but unless those without such abilities have a lower reproductive success or fitness, there is no barrier to its degeneration.
Behavioral

Humans also bear some vestigial behaviors and reflexes. For example, the formation of goose bumps in humans under stress is a vestigial reflex;[52] a possible function in human evolutionary ancestors was to raise the body's hair, making the ancestor appear larger and scaring off predators. Raising the hair is also used to trap an extra layer of air, keeping an animal warm. Due to the diminished amount of hair in humans, the reflex formation of goose bumps when cold is also vestigial.
The palmar grasp reflex is supported to be a vestigial behavior in human infants. When placing a finger or object to the palm of an infant, it will securely grasp it. This grasp is found to be rather strong.[53] Some infants—37% according to a 1932 study—are able to support their own weight from a rod,[54] although there is no way they can cling to their mother. The grasp is also evident in the feet too. When a baby is sitting down, its prehensile feet assume a curled-in posture, similar to that observed in an adult chimp.[55][56] An ancestral primate would have had sufficient body hair to which an infant could cling unlike modern humans, thus allowing its mother to escape from danger, such as climbing up a tree in the presence of a predator without having to occupy her hands holding her baby.
It has been proposed that the hiccup is an evolutionary remnant of earlier amphibian respiration.[57] Amphibians such as tadpoles gulp air and water across their gills via a rather simple motor reflex akin to mammalian hiccuping. The motor pathways that enable hiccuping form early during fetal development, before the motor pathways that enable normal lung ventilation form. Thus, according to recapitulation theory the hiccup is evolutionarily antecedent to modern lung respiration. Additionally, they point out that hiccups and amphibian gulping are inhibited by elevated CO2 and may be stopped by GABAB receptor agonists, illustrating a possible shared physiology and evolutionary heritage. These proposals may explain why premature infants spend 2.5% of their time hiccuping, possibly gulping like amphibians, as their lungs are not yet fully formed. Fetal intrauterine hiccups are of two types. The physiological type occurs prior to twenty-eight weeks after conception and tend to last five to ten minutes. These hiccups are part of fetal development and are associated with the myelination of the phrenic nerve, which primarily controls the thoracic diaphragm. The phylogeny hypothesis explains how the hiccup reflex might have evolved, and if there is not an explanation it may explain hiccups as an evolutionary remnant, held-over from our amphibious ancestors. This hypothesis has been questioned because of the existence of the afferent loop of the reflex, the fact that it does not explain the reason for glottic closure, and because the very short contraction of the hiccup is unlikely to have a significant strengthening effect on the slow-twitch muscles of respiration.
Molecular
There are also vestigial molecular structures in humans, which are no longer in use but may indicate common ancestry with other species. One example of this is L-gulonolactone oxidase, a gene that is functional in most other mammals and produces an enzyme that synthesizes vitamin C.[58] In humans and other members of the suborder Haplorrhini, a mutation disabled the gene and made it unable to produce the enzyme. However, the remains of the gene are still present in the human genome as a vestigial genetic sequence called a pseudogene.[59]
See also
References
- ↑ Wiedersheim, Robert (1893). The Structure of Man: an index to his past history. London: Macmillan and Co.
- ↑ Wiedersheim, Robert Ernst Eduard. The structure of man an index to his past history. Macmillan 1895. May be downloaded from
- ↑ Muller, G. B. (2002) "Vestigial Organs and Structures." in Encyclopedia of Evolution. Mark Pagel, editor in chief, New York: Oxford University Press. pp. 1131–1133.
- ↑ Koerth-Baker, Maggie (30 July 2009). "Vestigial Organs Not So Useless After All". National Geographic. Retrieved 27 July 2013.
- ↑ 5.0 5.1 Wells, H.g. Huxley, J. Wells, G. P. The Science of Life. Pub. Cassell 1931
- ↑ Rosenthal, M. I.: Journal of the American Medical Association, Volume 67, Issues 15-26, 1916. Page 1326
- ↑ W. Colin MacKenzie. A Contribution to the Biology of the Vermiform Appendix. Medical record, Volume 89 Page 342 1916
- ↑ 8.0 8.1 8.2 8.3 8.4 Darwin, Charles (1871). The Descent of Man, and Selection in Relation to Sex. John Murray: London.
- ↑ Stevens, C. Edward; Hume, Ian (2004). Comparative Physiology of the Vertebrate Digestive System. Cambridge: Cambridge University Press. ISBN 9780521617147.
- ↑ Peter Robert Cheeke, Ellen S. Dierenfeld, Comparative Animal Nutrition and Metabolism. Publisher: CABI; 2010 ISBN 978-1845936310
- ↑ Useful Appendix
- ↑ Randal Bollinger, R.; Barbas, Andrew S.; Bush, Errol L.; Lin, Shu S.; Parker, William (2007). "Biofilms in the large bowel suggest an apparent function of the human vermiform appendix". Journal of Theoretical Biology 249 (4): 826–31. doi:10.1016/j.jtbi.2007.08.032. PMID 17936308.
- ↑ Charles Q. Choi, "The Appendix: Useful and in Fact Promising", Live Science, 2009, Appendix has useful function
- ↑ Smith H. F., Fisher R. E., Everett M. L., Thomas A. D., Bollinger, R. R., Parker W. (2009). "Comparative anatomy and phylogenetic distribution of the mammalian cecal appendix". Journal of Evolutionary Biology 22 (10): 1984–1999. doi:10.1111/j.1420-9101.2009.01809.x. PMID 19678866.
- ↑ Saraga-Babić, M; Lehtonen, E; Svajger, A; Wartiovaara, J (1994). "Morphological and immunohistochemical characteristics of axial structures in the transitory human tail". Annals of Anatomy 176 (3): 277–86. doi:10.1016/S0940-9602(11)80496-6. PMID 8059973.
- ↑ Fallon, John F.; Simandl, B. Kay (1978). "Evidence of a role for cell death in the disappearance of the embryonic human tail". American Journal of Anatomy 152 (1): 111–29. doi:10.1002/aja.1001520108. PMID 677043.
- ↑ Dao, Anh H.; Netsky, Martin G. (1984). "Human tails and pseudotails". Human Pathology 15 (5): 449–53. doi:10.1016/S0046-8177(84)80079-9. PMID 6373560.
- ↑ Dubrow, Terry J.; Wackym, Phillip Ashley; Lesavoy, Malcolm A. (1988). "Detailing the Human Tail". Annals of Plastic Surgery 20 (4): 340–4. doi:10.1097/00000637-198804000-00009. PMID 3284435.
- ↑ Spiegelmann, Roberto (1985). "The human tail: a benign stigma Case report". Journal of Neurosurgery 63: 461–462. doi:10.3171/jns.1985.63.3.0461. PMID 3894599.
- ↑ Johnson, Dr. George B. "Evidence for Evolution". (Page 12) Txtwriter Inc. 8 Jun 2006.
- ↑ Rozkovcová, E; Marková, M; Dolejsí, J (1999). "Studies on agenesis of third molars amongst populations of different origin". Sbornik lekarsky 100 (2): 71–84. PMID 11220165.
- ↑ Pereira, T. V.; Salzano, FM; Mostowska, A; Trzeciak, WH; Ruiz-Linares, A; Chies, JA; Saavedra, C; Nagamachi, C; Hurtado, AM; Hill, K.; Castro-De-Guerra, D.; Silva-Junior, W. A.; Bortolini, M.-C. (2006). "Natural selection and molecular evolution in primate PAX9 gene, a major determinant of tooth development". Proceedings of the National Academy of Sciences 103 (15): 5676–81. doi:10.1073/pnas.0509562103. PMC 1458632. PMID 16585527.
- ↑ Trotier, D.; Eloit, C; Wassef, M; Talmain, G; Bensimon, JL; Døving, KB; Ferrand, J (2000). "The Vomeronasal Cavity in Adult Humans". Chemical Senses 25 (4): 369–80. doi:10.1093/chemse/25.4.369. PMID 10944499.
- ↑ Kjær, Inger; Fischer Hansen, Birgit (1996). "The human vomeronasal organ: prenatal developmental stages and distribution of luteinizing hormone-releasing hormone". European Journal of Oral Sciences 104 (1): 34–40. doi:10.1111/j.1600-0722.1996.tb00043.x. PMID 8653495.
- ↑ Smith, Timothy D.; Siegel, Michael I.; Bhatnagar, Kunwar P. (2001). "Reappraisal of the vomeronasal system of catarrhine primates: Ontogeny, morphology, functionality, and persisting questions". The Anatomical Record 265 (4): 176–92. doi:10.1002/ar.1152. PMID 11519019.
- ↑ Smith, Timothy D.; Bhatnagar, Kunwar P. (2000). "The human vomeronasal organ. Part II: prenatal development". Journal of Anatomy 197 (3): 421–36. doi:10.1046/j.1469-7580.2000.19730421.x. PMC 1468143. PMID 11117628.
- ↑ Won, J; Mair, EA; Bolger, WE; Conran, RM (2000). "The vomeronasal organ: an objective anatomic analysis of its prevalence". Ear, nose, & throat journal 79 (8): 600–5. PMID 10969469.
- ↑ Johnson, A; Josephson, R; Hawke, M (1985). "Clinical and histological evidence for the presence of the vomeronasal (Jacobson's) organ in adult humans". The Journal of otolaryngology 14 (2): 71–9. PMID 4068105.
- ↑ Foltán, René; Šedý, Jiří (2009). "Behavioral changes of patients after orthognathic surgery develop on the basis of the loss of vomeronasal organ: a hypothesis". Head & Face Medicine 5: 5. doi:10.1186/1746-160X-5-5. PMC 2653472. PMID 19161592.
- ↑ Bhatnagar, Kunwar P.; Smith, Timothy D. (2001). "The human vomeronasal organ. III. Postnatal development from infancy to the ninth decade". Journal of Anatomy 199 (Pt 3): 289–302. doi:10.1046/j.1469-7580.2001.19930289.x. PMC 1468331. PMID 11554506.
- ↑ 31.0 31.1 31.2 Bhatnagar, Kunwar P.; Kennedy, Ray C.; Baron, Georg; Greenberg, Richard A. (1987). "Number of mitral cells and the bulb volume in the aging human olfactory bulb: A quantitative morphological study". The Anatomical Record 218 (1): 73–87. doi:10.1002/ar.1092180112. PMID 3605663.
- ↑ Witt, M; Hummel, T (2006). "Vomeronasal Versus Olfactory Epithelium: Is There a Cellular Basis for Human Vomeronasal Perception?". International Review of Cytology. International Review of Cytology 248: 209–59. doi:10.1016/S0074-7696(06)48004-9. ISBN 9780123646521. PMID 16487792.
- ↑ Wysocki CJ, Preti G (November 2004). "Facts, fallacies, fears, and frustrations with human pheromones". The Anatomical Record. Part a, Discoveries in Molecular, Cellular, and Evolutionary Biology 281 (1): 1201–11. doi:10.1002/ar.a.20125. PMID 15470677.
- ↑ Wyatt, Tristram D. (2003). Pheromones and Animal Behaviour: Communication by Smell and Taste. Cambridge: Cambridge University Press. p. 295. ISBN 0-521-48526-6.
- ↑ Prof. A. Macalister, Annals and Magazine of Natural History, vol. vii., 1871, p. 342.
- ↑ Bair, J.H (1901); "Development of voluntary control", in Psychological Review, vol. 8, pp. 474-510.
- ↑ Mr. St. George Mivart, Elementary Anatomy, 1873, p. 396.
- ↑ Owen, R. 1866–1868. Comparative Anatomy and Physiology of Vertebrates. London.
- ↑ Montagna, W.; Machida, H.; Perkins, E.M. (1966). "The skin of primates XXXIII.: The skin of the angwantibo". American Journal of Physical Anthropology 25 (3): 277–290. doi:10.1002/ajpa.1330250307. PMID 5971502.
- ↑ Battista, J.; Zimmer, L.; Vazquez, J. (2011). "Muller’s Muscle, No Longer Vestigial in Endoscopic Surgery". World Neurosurgery 76 (3): 342–346. doi:10.1016/j.wneu.2010.12.057. PMID 21986434.
- ↑ Blank, Hanne (2007). Virgin: The Untouched History. Bloomsbury Publishing. pp. 23. ISBN 1-59691-010-0
- ↑ Blackledge, Catherine (2004). The Story of V. Rutgers University Press. ISBN 0-8135-3455-0.
Hymens, or vaginal closure membranes or vaginal constrictions, as they are often referred to, are found in a number of mammals, including llamas, ...
- ↑ Macalister A (1875). "Observations on muscular anomalies in the human anatomy. Third series with a catalogue of the principal muscular variations hitherto published". Trans. Roy. Irish Acad Sci 25: 1–130.
- ↑ Guerra, A. B.; Metzinger, SE; Metzinger, RC; Xie, C; Xie, Y; Rigby, PL; Naugle Jr, T (2004). "Variability of the Postauricular Muscle Complex: Analysis of 40 Hemicadaver Dissections". Archives of Facial Plastic Surgery 6 (5): 342–7. doi:10.1001/archfaci.6.5.342. PMID 15381582.
- ↑ Tamatsu, Y; Tsukahara, K; Hotta, M; Shimada, K (2007). "Vestiges of vibrissal capsular muscles exist in the human upper lip". Clinical Anatomy 20 (6): 628–31. doi:10.1002/ca.20497. PMID 17458869.
- ↑ Kapoor, SK; Tiwari, A; Kumar, A; Bhatia, R; Tantuway, V; Kapoor, S (2008). "Clinical relevance of palmaris longus agenesis: common anatomical aberration". Anatomical science international 83 (1): 45–8. doi:10.1111/j.1447-073X.2007.00199.x. PMID 18402087.
- ↑ Sebastin, SJ; Lim, AY; Bee, WH; Wong, TC; Methil, BV (2005). "Does the absence of the palmaris longus affect grip and pinch strength?". Journal of hand surgery (Edinburgh, Scotland) 30 (4): 406–8. doi:10.1016/j.jhsb.2005.03.011. PMID 15935531.
- ↑ Rubinstein, David; Escott, Edward J.; Hendrick, Laura L. (April 1999). "The prevalence and CT appearance of the levator claviculae muscle: a normal variant not to be mistaken for an abnormality". AJNR Am J Neuroradiol (American Society of Neuroradiology) 20 (4): 583–6. PMID 10319965.
- ↑ Loukas, M.; Sullivan, A.; Tubbs, R.S.; Shoja, M.M. (2008). "Levator claviculae: a case report and review of the literature". Folia Morphol. 67 (4): 307–310.
- ↑ Lovering, RM; Anderson, LD (2008). "Architecture and fiber type of the pyramidalis muscle". Anatomical science international 83 (4): 294–7. doi:10.1111/j.1447-073X.2007.00226.x. PMC 3531545. PMID 19159363.
- ↑ Ogata, S; Mine, K (2002). "Morphological study of the human chondroglossus muscle in Japanese". ANNALS OF ANATOMY 184: 493–499. doi:10.1016/S0940-9602(02)80087-5. PMID 12392330.
- ↑ Darwin, Charles. (1872) The Expression of the Emotions in Man and Animals John Murray, London.
- ↑ Peter Gray (2007). Psychology (fifth ed.). Worth Publishers. p. 66. ISBN 0-7167-0617-2.
- ↑ Behavior Development in Infants (via Google Books) by Evelyn Dewey, citing a study "Reflexes and other motor activities in newborn infants: a report of 125 cases as a preliminary study of infant behavior" published in the Bull. Neurol. Inst. New York, 1932, Vol. 2, pp. 1–56.
- ↑ Jerry Coyne (2009). Why Evolution is True. Penguin Group. pp. 85–86. ISBN 9780670020539.
- ↑ Anthony Stevens (1982). Archetype: A Natural History of the Self. Routledge & Kegan Paul. p. 87. ISBN 0-7100-0980-1.
- ↑ Straus, C.; Vasilakos, K; Wilson, RJ; Oshima, T; Zelter, M; Derenne, JP; Similowski, T; Whitelaw, WA (February 2003). "A phylogenetic hypothesis for the origin of hiccough". BioEssays 25 (2): 182–188. doi:10.1002/bies.10224. PMID 12539245.
- ↑ Ohta, Y; Nishikimi, M (1999). "Random nucleotide substitutions in primate nonfunctional gene for L-gulono-gamma-lactone oxidase, the missing enzyme in L-ascorbic acid biosynthesis". Biochimica et Biophysica Acta 1472 (1–2): 408–11. doi:10.1016/S0304-4165(99)00123-3. PMID 10572964.
- ↑ Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K (May 6, 1994). "Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man". J. Biol. Chem. 269 (18): 13685–8. PMID 8175804.
Further reading
| Wikisource has original text related to this article: |
- Shubin, Neil (2009). Your Inner Fish: A Journey into the 3.5-Billion-Year History of the Human Body. New York: Vintage Books. ISBN 0307277453.

