Human microbiota
The human microbiota is the aggregate of microorganisms, a microbiome that resides on the surface and in deep layers of skin (including in mammary glands),[1] in the saliva and oral mucosa, in the conjunctiva, and in the gastrointestinal tracts. They include bacteria, fungi, and archaea. Micro-animals which live on the human body are excluded. The human microbiome refer to their genomes.
One study indicated they outnumber human cells 10 to 1.[2] Some of these organisms perform tasks that are useful for the human host. However, the majority have been too poorly researched for us to understand the role they play, however communities of microflora have been shown to change their behavior in diseased individuals.[2] Those that are expected to be present, and that under normal circumstances do not cause disease, but instead participate in maintaining health, are deemed members of the normal flora. Though widely known as "microflora", this is, in technical terms, a misnomer, since the word root "flora" pertains to plants, and biota refers to the total collection of organisms in a particular ecosystem. Recently, the more appropriate term "microbiota" is applied, though its use has not eclipsed the entrenched use and recognition of "flora" with regard to bacteria and other microorganisms. Both terms are being used in different literature
Studies in 2009 questioned whether the decline in biota (including microfauna) as a result of human intervention might impede human health.[3] Most of the microbes associated with humans appear to be not harmful at all, but rather assist in maintaining processes necessary for a healthy body. A surprising finding was that at specific sites on the body, a different set of microbes may perform the same function for different people. For example, on the tongues of two people, two entirely different sets of organisms will break down sugars in the same way. This suggests that medical science may be forced to abandon the "one only" microbe model of infectious disease, and rather pay attention to functions of groups of microbes that have somehow gone awry.[4]
Bacteria
Populations of microbes (such as bacteria and yeasts) inhabit the skin and mucosal surfaces in various parts of the body. Their role forms part of normal, healthy human physiology, however if microbe numbers grow beyond their typical ranges (often due to a compromised immune system) or if microbes populate (such as through poor hygiene or injury) areas of the body normally not colonized or sterile (such as the blood, or the lower respiratory tract, or the abdominal cavity), disease can result (causing, respectively, bacteremia/sepsis, pneumonia, and peritonitis).
In 2012, some 200 researchers from some 80 research institutions comprising the Human Microbiome Project (HMP) Consortium have used advanced DNA-sequencing to identify and catalogue the thousands of microorganisms co-existing with humans. This study examined, amongst other things, the carbohydrate active enzymes from microbial populations from twelve sites on and in the human body, and concluded that microbes colonise each site to utilise the available sugars. Considerable variation was found in the enzymes for carbohydrate metabolism from site to site, and the researchers suggested that the composition of local carbohydrate metabolites may be the most important factor shaping the composition of microbial sub-communities of the human microbiome.
The same project examined the diversity of microbial communities present in multiple sites on the human body, using some 200 healthy persons and examining 18 sites on the body. Healthy individuals were found to host thousands of bacterial types, different body sites having their own distinctive communities. Skin and vaginal sites showed smaller diversity than the mouth and gut, these showing the greatest richness. The bacterial makeup for a given site on a body varies from person to person, not only in type, but also in abundance. Bacteria of the same species found throughout the mouth are of multiple subtypes, preferring to inhabit distinctly different locations in the mouth. Even the enterotypes in the human gut, previously thought to be well-understood, are from a broad spectrum of communities with blurred taxon boundaries.[5][6]
It is estimated that 500 to 1,000 species of bacteria live in the human gut[7][8] Bacterial cells are much smaller than human cells, and there are at least ten times as many bacteria as human cells in the body (approximately 1014 versus 1013).[9][10] The mass of microorganisms are estimated to account for 1-3% total body mass.[11] Though members of the flora are found on all surfaces exposed to the environment (on the skin and eyes, in the mouth, nose, small intestine), the vast majority of bacteria live in the large intestine.
Many of the bacteria in the digestive tract, collectively referred to as the gut flora, are able to break down certain nutrients such as carbohydrates that humans otherwise could not digest. The majority of these commensal bacteria are anaerobes, meaning they survive in an environment with no oxygen. Normal flora bacteria can act as opportunistic pathogens at times of lowered immunity.[12]
Escherichia coli (a.k.a. E. coli) is a bacterium that lives in the colon; it is an extensively studied model organism and probably the best-understood bacterium of all.[13] Certain mutated strains of these gut bacteria do cause disease; an example is E. coli O157:H7.
A number of types of bacteria, such as Actinomyces viscosus and A. naeslundii, live in the mouth, where they are part of a sticky substance called plaque. If this is not removed by brushing, it hardens into calculus (also called tartar). The same bacteria also secrete acids that dissolve tooth enamel, causing tooth decay.
The vaginal microflora consist mostly of various lactobacillus species. It was long thought that the most common of these species was Lactobacillus acidophilus, but it has later been shown that the most common one is L. iners followed by L. crispatus. Other lactobacilli found in the vagina are L. jensenii, L. delbruekii and L. gasseri. Disturbance of the vaginal flora can lead to infections such as bacterial vaginosis or candidiasis ("yeast infection").
Archaea
Archaea are present in the human gut, but, in contrast to the enormous variety of bacteria in this organ, the numbers of archaeal species are much more limited.[14] The dominant group are the methanogens, particularly Methanobrevibacter smithii and Methanosphaera stadtmanae.[15] However, colonization by methanogens is variable, and only about 50% of humans have easily detectable populations of these organisms.[16]
As of 2007, no clear examples of archaeal pathogens are known,[17][18] although a relationship has been proposed between the presence of some methanogens and human periodontal disease.[19]
Fungi
Fungi, in particular yeasts, are present in the human gut. The best-studied of these are Candida species. This is because of their ability to become pathogenic in immunocompromised hosts.[20] Yeasts are also present on the skin, particularly Malassezia species, where they consume oils secreted from the sebaceous glands.[21][22]
Acari

Among the biota living on human skin are two face mites, namely Demodex folliculorum and Demodex brevis, which reside in the sebaceous glands and hair follicles. These mites are of size range of 0.15 to 0.4 millimetres (0.0059 to 0.0157 in) depending upon the species, and can be detected by the naked eye as tiny dots; however, microscopes are required to distinguish the morophological characteristics. While these face mites may be found on others parts of the body, they predominantly occur on the face, especially around the nose, the eyebrows and the eyelashes. These two Demodex face-mite species are obligate human saprophytic ecto-parasites which feed on skin cells, hormones and oils. Presence of mites is usually asymptomatic and does not imply human Demodicosis, a disease caused by the face-mites and which is "considered as a multi-factorial disease, influenced by external and/or internal factors". Presence of mites in different groups of people have been found to range from 23% to 100%.[23]
Anatomical areas
Skin flora
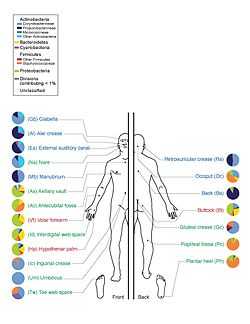
A study of twenty skin sites on each of ten healthy humans found 205 identified genera in nineteen bacterial phyla, with most sequences assigned to four phyla: Actinobacteria (51.8%), Firmicutes (24.4%), Proteobacteria (16.5%), and Bacteroidetes (6.3%).[24]
Microbial colonization in the human body begins shortly after birth, and average adults possess 10 times more microbial cells than human cells.[25] The skin acts as a barrier to deter the invasion of pathogenic bacteria. The human skin contains microbes that reside either in or on the skin and can be residential or transient.[25] Resident microorganism types vary in relation to skin type on the human body. A majority of bacteria reside on superficial cells on the skin or prefer to associate with glands.[25] These glands such as oil or sweat glands provide the bacteria with water, amino acids, and fatty acids that provide nutrients for the microbes.[25] In addition, resident bacteria can be pathogenic and are characteristically gram positive bacteria. Certain gram positive bacteria can be associated with oil glands that play a role in acne and skin disease.[25] Moreover, human sweat is by nature odorless, but bacteria associated with the skin play a role in producing body odor.[26] Researchers at Wageningen University in Netherlands discovered that humans with a large number of bacteria that possess a low level of diversity are more attractive to a particular species of mosquito.[26] The experiments were conducted with Anopheles gambiae sensu stricto mosquito, which are associated with malaria.[26]
Conjunctival flora
These are sparse in occurrence, but Gram-positive cocci and Gram-negative rods are present.[27]
A small number of bacteria are normally present in the conjunctiva. Staphylococcus epidermidis and certain coryneforms such as Propionibacterium acnes are dominant. Staphylococcus aureus, streptococci, Haemophilus sp. and Neisseria sp. sometimes occur. The lachrymal glands continuously secrete, keeping the conjunctiva moist, while intermittent blinking lubricates the conjunctiva and washes away foreign material. Tears contain bactericides such as lysozyme, so that microorganisms have difficulty in surviving the lysozyme and settling on the epithelial surfaces.
Some pathogens able to infect the conjunctiva, such as Neisseria gonorrhoeae and Chlamydia trachomatis are thought to have special processes allowing them to attach to the conjunctival epithelium. Newborn infants are particularly prone to bacterial attachment. Chlamydia and Neisseria may be present in an infected mother and show up on the cervical and vaginal epithelium - in such cases the newborn's eyes may be treated with silver nitrate or antibiotics.
Gut flora
The gut flora is the human flora of microorganisms that normally live in the digestive tract and can perform a number of useful functions for their hosts. The bacterial flora of the human gut encompasses a wide variety of microorganisms that aid in digestion, the synthesis of vitamins, and creating enzymes not produced by the human body.[25] According to scientific research, the human gut consists of different enterotypes that have an inconspicuous impact on human health.[28] It is suggested that the intestines of infants are colonized by bacteria that alter the gut to support those specific bacteria.[28] The average human body, consisting of about ten trillion cells, has about ten times that number of microorganisms in the gut.[7][29][30][31] The metabolic activity performed by these bacteria is equal to that of a virtual organ, leading to gut bacteria being termed a "forgotten" organ.[32]
Stomach flora
Due to the high acidity of the stomach, most microorganisms cannot survive. The main bacterial inhabitants of the stomach include: Streptococcus, Staphylococcus, Lactobacillus, Peptostreptococcus, and types of yeast.[25] Helicobacter pylori is a Gram-negative spiral organism that establishes on gastric mucosa causing chronic gastritis and peptic ulcer disease.[33] H. pylori has also been classified as a carcinogen for gastric cancer.[33]
Intestinal flora
The small intestine contains a trace amount of microorganisms due to the proximity and influence of the stomach. Gram positive cocci and rod shaped bacteria are the predominant microorganisms found in the small intestine.[25] However, in the distal portion of the small intestine alkaline conditions support gram-positive bacteria of the Enterobacteriaceae.[25] The bacterial flora of the small intestine aid in a wide range of intestinal functions. The bacterial flora provide regulatory signals that enable the development and utility of the gut. Overgrowth of bacteria in the small intestine can lead to intestinal failure. [34] In addition the large intestine contains the largest bacterial ecosystem in the human body.[25] Factors that disrupt the microorganism population of the large intestine include antibiotics, stress, and parasites.[25]
Bacteria make up most of the flora in the colon[35] and 60% of the dry mass of feces.[30] This fact makes feces an ideal source to test for gut flora for any tests and experiments by extracting the nucleic acid from fecal specimens, and bacterial 16S rRNA gene sequences are generated with bacterial primers. This form of testing is also often preferable to more invasive techniques, such as biopsies. Somewhere between 300[30] and 1000 different species live in the gut,[7] with most estimates at about 500.[31][36] However, it is probable that 99% of the bacteria come from about 30 or 40 species.[37] Fungi and protozoa also make up a part of the gut flora, but little is known about their activities.
Research suggests that the relationship between gut flora[38] and humans is not merely commensal (a non-harmful coexistence), but rather is a mutualistic, symbiotic relationship.[7] Though people can survive with no gut flora,[31] the microorganisms perform a host of useful functions, such as fermenting unused energy substrates, training the immune system, preventing growth of harmful species,[30] regulating the development of the gut, producing vitamins for the host (such as biotin and vitamin K), and producing hormones to direct the host to store fats. Extensive modification and imbalances of the gut microbiota and its microbiome or gene collection are associated with obesity.[39] However, in certain conditions, some species are thought to be capable of causing disease by causing infection or increasing cancer risk for the host.[30][35]
| Bacteria commonly found in the human colon[40] | |
| Bacterium | Incidence (%) |
|---|---|
| Bacteroides fragilis | 100 |
| Bacteroides melaninogenicus | 100 |
| Bacteroides oralis | 100 |
| Enterococcus faecalis | 100 |
| Escherichia coli | 100 |
| Enterobacter sp. | 40-80 |
| Klebsiella sp. | 40-80 |
| Bifidobacterium bifidum | 30-70 |
| Staphylococcus aureus | 30-50 |
| Lactobacillus | 20-60 |
| Clostridium perfringens | 25-35 |
| Proteus mirabilis | 5-55 |
| Clostridium tetani | 1-35 |
| Clostridium septicum | 5-25 |
| Pseudomonas aeruginosa | 3-11 |
| Salmonella enteritidis | 3-7 |
| Peptostreptococcus sp. | ?common |
| Peptococcus sp. | ?common |
Vaginal flora
The most abundant vaginal microorganism found in premenopausal women is Lactobacillus bacteria.[41] Vaginal flora is influenced by a variety of factors including exogenous and endogenous influences.[41] Bacteria type vary in women depending on the stage of the menstrual cycle.[42] Lactic acid bacteria are predominately found during child-bearing years, otherwise the bacterial flora is mixed.[42] Although the menstrual cycle alters vaginal pH and the growth of various organisms, some research shows lactobacilli remain at a constant level regardless of the stage of menstruation[41] while other research shows that during menstruation, the concentration of vaginal microbiome is observed to decline.[43] Race also influences vaginal flora. The occurrence of hydrogen peroxide-producing lactobacilli is lower in African American women, and vaginal pH is higher.[41] Other influential factors such as sexual intercourse and antibiotics have been linked to the loss of lactobacilli.[44] Moreover, studies have found that sexual intercourse with a condom does appear to change lactobacilli levels, and does increase the level of Escherichia coli within the vaginal flora.[44] Disruption of vaginal flora can lead to infections such as vaginal candidiasis or bacterial vaginosis (BV).[44]
Oral cavity
The human mouth is an ideal environment of the existence and growth of microorganisms. It provides a source of water and nutrients, as well as a moderate temperature.[25] Resident bacteria of the mouth adhere to the teeth and gums to resist mechanical flushing from the mouth to stomach where they are destroyed by hydrochloric acid.[25] Anaerobic bacteria in the oral cavity include: Actinomyces, Arachnia, Bacteroides, Bifidobacterium, Eubacterium, Fusobacterium, Lactobacillus, Leptotrichia, Peptococcus, Peptostreptococcus, Propionibacterium, Selenomonas, Treponema, and Veillonella.[45]
Respiratory flora
Much like the oral cavity, the upper and lower respiratory system possess mechanical deterrents to remove bacteria. Goblet cells produce mucous which traps bacteria and moves them out of the respiratory system via continuously moving ciliated epithelial cells.[25] In addition, a bactericidal effect is generated by nasal mucus which contains the enzyme lysozyme.[25]
Nonetheless, the upper and lower respiratory tract appears to have a normal bacterial flora. A significant portion of the normal biota belongs to 9 major bacterial genera: Prevotella, Sphingomonas, Pseudomonas, Acinetobacter, Fusobacterium, Megasphaera, Veillonella, Staphylococcus, and Streptococcus. Note that some bacteria considered "normal biota" in the respiratory tract can cause serious disease especially in immunocompromised individuals; these include Streptococcus pyogenes, Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Staphylococcus aureus.
Unusual bacterial flora in the respiratory system can be detrimental and has been seen in patients with cystic fibrosis[46] The bacterial flora found in the lungs of patients with cystic fibrosis often contains antibiotic-resistant and slow-growing bacteria, and the frequency of these pathogens changes in relation to age.[46]
See also
- Microbiome
- Microorganism
- Human Microbiome Project
- Drug resistance
- List of human diseases associated with infectious pathogens
- Initial acquisition of microbiota
References
- ↑ http://www.sciencedaily.com/releases/2013/01/130104083103.htm
- ↑ 2.0 2.1 http://medicalxpress.com/news/2014-08-mouth-bacteria-diet-supercomputers-reveal.html
- ↑ Katherine Harmon (16 December 2009). "Bugs Inside: What Happens When the Microbes That Keep Us Healthy Disappear?". Scientific American. Retrieved 27 December 2008.
- ↑ "Human Microbiome Project reveals largest microbial map". BBC News. 13 June 2012.
- ↑ http://www.plos.org/media/press/2012/PLoS_%20HMP_Collection_Manuscript_Summaries.pdf
- ↑ Consortium of Scientists Map the Human Body’s Bacterial Ecosystem
- ↑ 7.0 7.1 7.2 7.3 Sears (May 2009). "Topographical and Temporal Diversity of the Human Skin Microbiome". Science 324 (5931): 1190–1192. doi:10.1126/science.1171700. PMC 2805064. PMID 19478181.
- ↑ Pappas S. (2009). Your Body Is a habitat ... for Bacteria. Science Now Daily News
- ↑ Savage, D. C. (1977). "Microbial Ecology of the Gastrointestinal Tract". Annual Review of Microbiology 31 (1): 107–33. doi:10.1146/annurev.mi.31.100177.000543. PMID 334036.
- ↑ Berg, R. (1996). "The indigenous gastrointestinal microflora". Trends in Microbiology 4 (11): 430–5. doi:10.1016/0966-842X(96)10057-3. PMID 8950812.
- ↑ MacDougall, Raymond (13 June 2012). "NIH Human Microbiome Project defines normal bacterial makeup of the body". NIH. Retrieved 2012-09-20.
- ↑ Samuel Baron MD; Charles Patrick. Davis (1996). "Bacteriology". University of Texas Medical Branch at Galveston. pp. Chapter 6. Normal Flora.
- ↑ Lee PS, Lee KH (2003). "Escherichia coli--a model system that benefits from and contributes to the evolution of proteomics.". Biotechnol Bioeng 84 (7): 801–14. doi:10.1002/bit.10848. PMID 14708121.
- ↑ Eckburg PB, Bik EM, Bernstein CN et al. (2005). "Diversity of the human intestinal microbial flora". Science 308 (5728): 1635–8. doi:10.1126/science.1110591. PMC 1395357. PMID 15831718.
- ↑ Duncan SH, Louis P, Flint HJ (2007). "Cultivable bacterial diversity from the human colon". Lett. Appl. Microbiol. 44 (4): 343–50. doi:10.1111/j.1472-765X.2007.02129.x. PMID 17397470.
- ↑ Florin TH, Zhu G, Kirk KM, Martin NG (2000). "Shared and unique environmental factors determine the ecology of methanogens in humans and rats". Am. J. Gastroenterol. 95 (10): 2872–9. doi:10.1111/j.1572-0241.2000.02319.x. PMID 11051362.
- ↑ Eckburg P, Lepp P, Relman D (2003). "Archaea and their potential role in human disease". Infection and Immunity 71 (2): 591–6. doi:10.1128/IAI.71.2.591-596.2003. PMC 145348. PMID 12540534.
- ↑ Cavicchioli R, Curmi P, Saunders N, Thomas T (2003). "Pathogenic archaea: do they exist?". BioEssays 25 (11): 1119–28. doi:10.1002/bies.10354. PMID 14579252.
- ↑ Lepp P, Brinig M, Ouverney C, Palm K, Armitage G, Relman D (2004). "Methanogenic Archaea and human periodontal disease". Proc. Natl. Acad. Sci. U.S.A. 101 (16): 6176–81. doi:10.1073/pnas.0308766101. PMC 395942. PMID 15067114.
- ↑ Bernhardt H, Knoke M (1997). "Mycological aspects of gastrointestinal microflora". Scand. J. Gastroenterol. Suppl. 222: 102–6. PMID 9145460.
- ↑ Marcon MJ, Powell DA (1 April 1992). "Human infections due to Malassezia spp". Clin. Microbiol. Rev. 5 (2): 101–19. PMC 358230. PMID 1576583.
- ↑ Roth RR, James WD (1988). "Microbial ecology of the skin". Annu. Rev. Microbiol. 42 (1): 441–64. doi:10.1146/annurev.mi.42.100188.002301. PMID 3144238.
- ↑ Rather, Parvaiz Anwar & Hassan, Iffat (2014). "Human Demodex Mite: The Versatile Mite of Dermatological Importance". Indian Journal of Dermatology 59 (1): 60–66. doi:10.4103/0019-5154.123498. Retrieved 13 March 2015.
- ↑ Grice, Elizabeth A.; Kong, HH; Conlan, S; Deming, CB; Davis, J; Young, AC; NISC Comparative Sequencing Program; Bouffard, GG et al. (2006). "Topographical and Temporal Diversity of the Human Skin Microbiome". Science 324 (5931): 1190–2. doi:10.1126/science.1171700. PMC 2805064. PMID 19478181.
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 25.7 25.8 25.9 25.10 25.11 25.12 25.13 25.14 Willey, Joanne; Sherwood, Linda; Woolverton, Christopher (2011). Prescott's Microbiology (8th ed.). New York: McGraw Hill. pp. 731–737. ISBN 9780077350130. LCCN 2009033823. OCLC 434613235.
- ↑ 26.0 26.1 26.2 Verhulst, N. O.; Qiu, Y. T.; Beijleveld, H.; Maliepaard, C.; Knights, D.; Schulz, S.; Berg-Lyons, D.; Lauber, C. L.; Verduijn, W.; Haasnoot, G. W.; Mumm, R.; Bouwmeester, H. J.; Claas, F. H.; Dicke, M.; Van Loon, J. J.; Takken, W.; Knight, R.; Smallegange, R. C. (2011). Schneider, Bradley S, ed. "Composition of Human Skin Microbiota Affects Attractiveness to Malaria Mosquitoes". PLoS ONE 6 (12): e28991. doi:10.1371/journal.pone.0028991. PMC 3247224. PMID 22216154.
- ↑ The Normal Bacterial Flora of Humans
- ↑ 28.0 28.1 Zimmer, Carl (2011-04-20). "Gut Bacteria Divide People Into 3 Types, Scientists Report". The New York Times. ISSN 0362-4331. Retrieved 2013-04-20.
- ↑ Björkstén, B; Sepp, E; Julge, K; Voor, T; Mikelsaar, M (2001). "Allergy development and the intestinal microflora during the first year of life". The Journal of Allergy and Clinical Immunology 108 (4): 516–520. doi:10.1067/mai.2001.118130. PMID 11590374.
- ↑ 30.0 30.1 30.2 30.3 30.4 Guarner, F; Malagelada, JR (2003). "Gut flora in health and disease.". Lancet 361 (9356): 512–9. doi:10.1016/S0140-6736(03)12489-0. PMID 12583961.
- ↑ 31.0 31.1 31.2 Steinhoff, U (2005). "Who controls the crowd? New findings and old questions about the intestinal microflora.". Immunology letters 99 (1): 12–6. doi:10.1016/j.imlet.2004.12.013. PMID 15894105.
- ↑ O'Hara, Ann M; Shanahan, Fergus (2006). "The gut flora as a forgotten organ". EMBO Reports 7 (7): 688–693. doi:10.1038/sj.embor.7400731. PMC 1500832. PMID 16819463.
- ↑ 33.0 33.1 Brendan Drumm, et al. "Is Helicobacter Pylori Infection In Childhood A Risk Factor For Gastric Cancer?." Pediatrics 107.2 (2001): 373. Education Research Complete.
- ↑ Quigley, Eamonn M M; Rodrigo Quera (February 2006). "Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics". Gastroenterology 130 (2 Suppl 1): S78–90. doi:10.1053/j.gastro.2005.11.046. ISSN 0016-5085. PMID 16473077.
- ↑ 35.0 35.1 University of Glasgow. 2005. The normal gut flora. Available through web archive. Accessed May 22, 2008
- ↑ Gibson, RG. (2004). "Fibre and effects on probiotics (the prebiotic concept)". Clinical Nutrition Supplements 1 (2): 25–31. doi:10.1016/j.clnu.2004.09.005.
- ↑ Beaugerie, L; Petit, JC. (2004). "Microbial-gut interactions in health and disease. Antibiotic-associated diarrhoea". Best Practice & Research Clinical Gastroenterology 18 (2): 337–352. doi:10.1016/j.bpg.2003.10.002. PMID 15123074.
- ↑ Gut flora are also known as gut microbiota.
- ↑ Ley, Ruth E. "Obesity and the Human Microbiome." Current Opinion in Gastroenterology 26.1 (2010): 5-11. Wolters Kluwer Health. doi: 10.1097/MOG.0b013e328333d751
- ↑ Normal Bacterial Flora of Humans
- ↑ 41.0 41.1 41.2 41.3 Antonio, M. A. D.; Hawes, S. E.; Hillier, S. L. (1999). "The Identification of VaginalLactobacillusSpecies and the Demographic and Microbiologic Characteristics of Women Colonized by These Species". The Journal of Infectious Diseases 180 (6): 1950–1956. doi:10.1086/315109. PMID 10558952.
- ↑ 42.0 42.1 Todar, Kenneth (2012). "The Normal Bacterial Flora of Humans". Todar's Online Textbook of Bacteriology. Madison, WI: Kenneth Todar. Retrieved 2012-04-06.
- ↑ Onderdonk, A. B.; Zamarchi, G. R.; Walsh, J. A.; Mellor, R. D.; Muñoz, A.; Kass, E. H. (1986). "Methods for quantitative and qualitative evaluation of vaginal microflora during menstruation". Applied and environmental microbiology 51 (2): 333–339. PMC 238869. PMID 3954346.
- ↑ 44.0 44.1 44.2 Witkin, S. S.; Linhares, I. M.; Giraldo, P. (2007). "Bacterial flora of the female genital tract: Function and immune regulation". Best Practice & Research Clinical Obstetrics & Gynaecology 21 (3): 347–354. doi:10.1016/j.bpobgyn.2006.12.004. PMID 17215167.
- ↑ Sutter, V. L. (1984). "Anaerobes as normal oral flora". Reviews of infectious diseases. 6 Suppl 1: S62–S66. doi:10.1093/clinids/6.Supplement_1.S62. PMID 6372039.
- ↑ 46.0 46.1 Beringer, P M; M D Appleman (November 2000). "Unusual respiratory bacterial flora in cystic fibrosis: microbiologic and clinical features" (PDF). Current Opinion in Pulmonary Medicine 6 (6): 545–550. doi:10.1097/00063198-200011000-00015. ISSN 1070-5287. PMID 11100967.