Hill equation (biochemistry)
In biochemistry, the binding of a ligand to a macromolecule is often enhanced if there are already other ligands present on the same macromolecule (this is known as cooperative binding). The Hill coefficient provides a way to quantify this effect.
It describes the fraction of the macromolecule saturated by ligand as a function of the ligand concentration; it is used in determining the degree of cooperativeness of the ligand binding to the enzyme or receptor. It was originally formulated by Archibald Hill in 1910 to describe the sigmoidal O2 binding curve of hemoglobin.[1]
A coefficient of 1 indicates completely independent binding, regardless of how many additional ligands are already bound. Numbers greater than one indicate positive cooperativity, while numbers less than one indicate negative cooperativity. The Hill coefficient of oxygen binding to hemoglobin is 2.3-3.0.
Hill equation:
![\theta = {[L]^n \over K_d + [L]^n} = {[L]^n \over (K_A)^n + [L]^n} = {1 \over ({K_A \over [L]})^n+1}](../I/m/f4df40135881aa3d64f6304948423c2f.png)
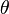 - fraction of the ligand-binding sites on the receptor protein which are occupied by the ligand.
- fraction of the ligand-binding sites on the receptor protein which are occupied by the ligand.
![[L]](../I/m/fc45d9d1939821a531d72be72d89eae4.png) - free (unbound) ligand concentration
- free (unbound) ligand concentration
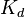 - Apparent dissociation constant derived from the law of mass action (equilibrium constant for dissociation)
- Apparent dissociation constant derived from the law of mass action (equilibrium constant for dissociation)
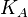 - ligand concentration producing half occupation (ligand concentration occupying half of the binding sites). This is also the microscopic dissociation constant. In recent literature, this constant is sometimes referred to as
- ligand concentration producing half occupation (ligand concentration occupying half of the binding sites). This is also the microscopic dissociation constant. In recent literature, this constant is sometimes referred to as 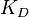 .
.
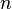 - Hill coefficient, describing cooperativity (or possibly other biochemical properties, depending on the context in which the Hill equation is being used)
- Hill coefficient, describing cooperativity (or possibly other biochemical properties, depending on the context in which the Hill equation is being used)
Taking the reciprocal of both sides, rearranging, inverting again, and then taking the logarithm on both sides of the equation leads to an alternative formulation of the Hill equation:
![\log\left( {\theta\over 1-\theta} \right) = n\log{[L]} - \log{K_A}.](../I/m/184631c4c87e670b515b6fd5dcb943b2.png)
When appropriate, the value of the Hill coefficient describes the cooperativity of ligand binding in the following way:
-
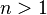 - Positively cooperative binding: Once one ligand molecule is bound to the enzyme, its affinity for other ligand molecules increases.
- Positively cooperative binding: Once one ligand molecule is bound to the enzyme, its affinity for other ligand molecules increases.
-
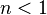 - Negatively cooperative binding: Once one ligand molecule is bound to the enzyme, its affinity for other ligand molecules decreases.
- Negatively cooperative binding: Once one ligand molecule is bound to the enzyme, its affinity for other ligand molecules decreases.
-
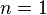 - Noncooperative binding: The affinity of the enzyme for a ligand molecule is not dependent on whether or not other ligand molecules are already bound. In this case, the Hill equation (as a relationship between the concentration of a compound adsorbing to binding sites and the fractional occupancy of the binding sites) is equivalent to the Langmuir equation.
- Noncooperative binding: The affinity of the enzyme for a ligand molecule is not dependent on whether or not other ligand molecules are already bound. In this case, the Hill equation (as a relationship between the concentration of a compound adsorbing to binding sites and the fractional occupancy of the binding sites) is equivalent to the Langmuir equation.
See also
The Hill equation is related to the logistic function and is in some ways a logarithmic transform of it, i.e. when you plot the Hill function on a log scale it looks identical to a logistic function. This is particularly important if the range of concentrations that results in saturation does not vary over several orders of magnitude. In such a case the logistic function would be a more appropriate equation to model the behavior.
- Logistic function
- Gompertz curve
- Sigmoid function
References
- ↑ Hill, A. V. (1910-01-22). "The possible effects of the aggregation of the molecules of hæmoglobin on its dissociation curves" (PDF). J. Physiol. 40 (Suppl): iv–vii. Retrieved 2009-03-18.
- Dorland's Illustrated Medical Dictionary
- Nelson, David L.; Cox, Michael M. Lehninger Principles of Biochemistry (4th ed.).
- Coval ML (December 1970). "Analysis of Hill interaction coefficients and the invalidity of the Kwon and Brown equation". J. Biol. Chem. 245 (23): 6335–6. PMID 5484812.
- d'A Heck, Henry (1971). "Statistical theory of cooperative binding to proteins. Hill equation and the binding potential". J. Am. Chem. Soc. 93 (1): 23–29. doi:10.1021/ja00730a004.
- Atkins, Gordon L. (1973). "A simple digital-computer program for estimating the parameter of the Hill Equation". Eur. J. Biochem. 33 (1): 175–180. doi:10.1111/j.1432-1033.1973.tb02667.x.
- Endrenyi, Laszlo; Kwong, F. H. F.; Fajszi, Csaba (1975). "Evaluation of Hill slopes and Hill coefficients when the saturation binding or velocity is not known". Eur. J. Biochem. 51 (2): 317–328. doi:10.1111/j.1432-1033.1975.tb03931.x.
- Voet, Donald; Voet, Judith G. Biochemistry.
- Weiss, J. N. (1997). "The Hill equation revisited: uses and misuses". FASEB 11 (11): 835–841. PMID 9285481.
- Kurganov, B. I.; Lobanov, A. V. (2001). "Criterion for Hill equation validity for description of biosensor calibration curves". Anal. Chimica Acta 427 (1): 11–19. doi:10.1016/S0003-2670(00)01167-3.
- Goutelle1, Sylvain; Maurin, Michel; Rougier, Florent; Barbaut, Xavier; Bourguignon, Laurent; Ducher, Michel; Maire, Pascal (2008). "The Hill equation: a review of its capabilities in pharmacological modelling". Fund. Clinic. Pharmac. 22: 633–648. doi:10.1111/j.1472-8206.2008.00633.x.
- Mitavskiy, B.; Chu, D.; Zabet., Radu (2009). "Models of transcription factor binding: Sensitivity of activation functions to model assumptions.". Journal of Theoretical Biology 257 (3): 419–429. doi:10.1016/j.jtbi.2008.11.026. PMID 19121637.
- Gesztelyi R; Zsuga J; Kemeny-Beke A; Varga B; Juhasz B; Tosaki A (2012). "The Hill equation and the origin of quantitative pharmacology". Archive for History of Exact Sciences 66: 427–38. doi:10.1007/s00407-012-0098-5.
- Colquhoun D (2006). "The quantitative analysis of drug-receptor interactions: a short history". Trends Pharmacol Sci 27 (3): 149–57. doi:10.1016/j.tips.2006.01.008. PMID 16483674.
- Rang HP (2006). "The receptor concept: pharmacology's big idea". Br J Pharmacol 147: S9–16.
- Neubig RR; Spedding M; Kenakin T; Christopoulos A (2003). "International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. update on terms and symbols in quantitative pharmacology". Pharmacol Rev 55: 597–606. doi:10.1124/pr.55.4.4.