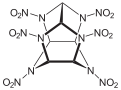
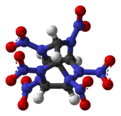
Hexanitrohexaazaisowurtzitane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane | |||
| Other names
CL-20 | |||
| Identifiers | |||
| Abbreviations | CL-20, HNIW | ||
| 135285-90-4 | |||
| ChEBI | CHEBI:77327 | ||
| ChemSpider | 8064994 9223599 (3R,9R)-dodec 9594121 (3R,5S,9R,11S)- dodec | ||
| |||
| Jmol-3D images | Image Image | ||
| PubChem | 9889323 11048432 (3R,9R)-dodec 11419235 (3R,5S,9R,11S)- dodec | ||
| |||
| Properties | |||
| C 6N 12H 6O 12 | |||
| Molar mass | 438.1850 g mol−1 | ||
| Density | 2.044 g cm−3 | ||
| Explosive data | |||
| Detonation velocity | 9.38 km s−1 | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Hexanitrohexaazaisowurtzitane /ˈhɛksɑːˈnaɪtroʊˈhɛksɑːˌæzɑːˌaɪsoʊˈvʊərtsɪteɪn/, also called HNIW and CL-20, is a nitroamine explosive with the formula C6H6N12O12, developed by the China Lake facility, primarily to be used in propellants. It has a better oxidizer-to-fuel ratio than conventional HMX or RDX. It produces 20% more energy than traditional HMX-based propellants, and is widely superior to conventional high-energy propellants and explosives.
While most development of CL-20 has been fielded by the Thiokol Corporation, the US Navy (through ONR) has also been interested in CL-20 for use in rocket propellants, such as for missiles, as it has lower observability characteristics such as less visible smoke.[1]
CL-20 has not yet been fielded in any production weapons system, but is undergoing testing for stability, production capabilities, and other weapons characteristics.
Synthesis
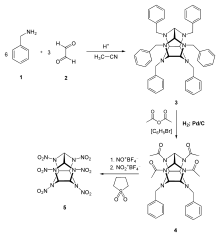
First, benzylamine (1) is condensed with glyoxal (2) under acidic and dehydrating conditions to yield the first intermediate compound, hexabenzylhexaazaisowurtzitane (3). Four benzyl groups selectively undergo hydrogenolysis using palladium on carbon and hydrogen. The amino groups are then acetylated during the same step using acetic anhydride as the solvent, yielding dibenzyltetraethanoylhexaazaisowurtzitane (4). Finally, compound 4 is reacted with nitronium tetrafluoroborate and nitrosonium tetrafluoroborate, resulting in HNIW.
Cocrystal product with HMX
In August 2012, Onas Bolton et al published results showing that a cocrystal of 2 parts CL-20 and 1 part HMX had similar safety properties to HMX, but with a greater firing power closer to CL-20. [2][3]
Cocrystal product with TNT
In August 2011, Adam Matzger and Onas Bolton published results showing that a cocrystal of CL-20 and TNT had twice the stability of CL-20—safe enough to transport, e.g. -- but by heating to 136 C can be separated into TNT and a form of CL-20 with structural defects that is somewhat less stable than CL-20.[4]
See also
- 2,4,6-Tris(trinitromethyl)-1,3,5-triazine
- 4,4’-Dinitro-3,3’-diazenofuroxan (DDF)
- Heptanitrocubane (HNC)
- HHTDD
- Octanitrocubane (ONC)
- Iceane (Wurtzitane)
- RE factor
References
- ↑ Yirka, Bob (9 September 2011). "University chemists devise means to stabilize explosive CL-20". Physorg.com. Retrieved 8 July 2012.
- ↑ High Power Explosive with Good Sensitivity: A 2:1 Cocrystal of CL-20:HMX, Crystal Growth & Design (American Chemical Society), 2012, 12 (9), pp 4311–4314, DOI: 10.1021/cg3010882, Publication Date (Web): August 7, 2012, accessed 7 September 2012
- ↑ Powerful new explosive could replace today's state-of-the-art military explosive, SpaceWar.com, 6 September 2012, accessed 7 September 2012
- ↑ Angewandte Chemie International Edition]
Further reading
- Bolton, Onas; Adam J. Matzger (September 12, 2011). "Improved Stability and Smart-Material Functionality Realized in an Energetic Cocrystal". Angewandte Chemie 123 (38): 9122–9125. doi:10.1002/ange.201104164. Retrieved 8 July 2012.
- Ehrenberg, Rachel (22 October 2011). "Explosive Goes Boom, But Not Too Soon". Science News 180 (9): 10. doi:10.1002/scin.5591800909. Retrieved 8 July 2012.
- Lowe, Derek (11 November 2011) "Things I won't work with: Hexanitrohexaazaisowurtzitane"