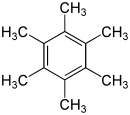
Hexamethylbenzene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,2,3,4,5,6-Hexamethylbenzene | |||
| Other names
Mellitene | |||
| Identifiers | |||
| 87-85-4 | |||
| ChEBI | CHEBI:39001 | ||
| ChemSpider | 6642 | ||
| |||
| Jmol-3D images | Image | ||
| PubChem | 6908 | ||
| |||
| Properties | |||
| Molecular formula |
C12H18 | ||
| Molar mass | 162.27 g·mol−1 | ||
| Appearance | White crystalline powder | ||
| Melting point | 165 °C (329 °F; 438 K) | ||
| Boiling point | 265.2 °C (509.4 °F; 538.3 K) | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Hexamethylbenzene is white crystalline solid with chemical formula C6(CH3)6. It is an aromatic hydrocarbon with six methyl groups stemming from the carbon centres of the ring. Hexamethylbenzene has historical significance in the field of X-ray crystallography. In 1929, Kathleen Lonsdale first proved the shape of hexamethylbenzene and thus showed that the benzene ring is hexagonal and flat.[1]
Uses
Hexamethylbenzene has no significant commercial uses. The six methyl groups enhance the proton affinity of the central ring.[2] Because it is electron-rich, hexamethylbenzene can be used as a ligand in organometallic chemistry. Two examples from organoruthenium chemistry are the sandwich complexes Ru(ɳ4-C6(CH3)6)(ɳ6-C6(CH3)6) and the dication [Ru(ɳ6-C6(CH3)6)2]2+.[3]
Hexamethylbenzene has been used as a solvent for 3He-NMR spectroscopy.[4]
Preparation
Hexamethylbenzene can be prepared by the reaction of phenol with methanol at 400 °C in the presence of an alumina catalyst.[5]
References
- ↑ Lonsdale, Kathleen (1929). "The Structure of the Benzene Ring in Hexamethylbenzene". Proceedings of the Royal Society A 123 (792): 494–515. doi:10.1098/rspa.1929.0081.
- ↑ Earhart., H. W.; Komin, Andrew P. (2000), "Polymethylbenzenes", Kirk-Othmer Encyclopedia of Chemical Technology, New York: John Wiley, doi:10.1002/0471238961.1615122505011808.a01, ISBN 9780471238966
- ↑ Bennett, M. A.; Huang, T. N.; Matheson, T. W.; Smith, A. K. (1982). "(η6-Hexamethylbenzene)Ruthenium Complexes". Inorganic Syntheses 21: 74–78. doi:10.1002/9780470132524.ch16. ISBN 9780470132524.
- ↑ Saunders, M.; Jimenez-Vazquez, H. A.; Khong, A. (1996). "NMR of 3He Dissolved in Organic Solids". J. Phys. Chem. 100 (39): 15968–15971. doi:10.1021/jp9617783.
- ↑ Landis, Phillip S.; Haag, Werner O. (1963). "Formation of Hexamethylbenzene from Phenol and Methanol". The Journal of Organic Chemistry 28 (2): 585. doi:10.1021/jo01037a517.