Heptane
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Heptane[1] | |
| Identifiers | |
| 1730763 | |
| 142-82-5 | |
| ChEBI | CHEBI:43098 |
| ChEMBL | ChEMBL134658 |
| ChemSpider | 8560 |
| EC number | 205-563-8 |
| 49760 | |
| |
| Jmol-3D images | Image |
| MeSH | n-heptane |
| PubChem | 8900 |
| RTECS number | MI7700000 |
| |
| UNII | 456148SDMJ |
| UN number | 1206 |
| Properties | |
| Molecular formula |
C7H16 |
| Molar mass | 100.20 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Petrolic |
| Density | 0.6795 g mL−1 |
| Melting point | −91.0 to −90.1 °C; −131.7 to −130.3 °F; 182.2 to 183.0 K |
| Boiling point | 98.1 to 98.7 °C; 208.5 to 209.6 °F; 371.2 to 371.8 K |
| 0.0003% (20°C)[2] | |
| log P | 4.274 |
| Vapor pressure | 5.33 kPa (at 20.0 °C) |
| Henry's law constant (kH) |
12 nmol Pa−1 kg−1 |
| Refractive index (nD) |
1.387 |
| Viscosity | 386 μPa s |
| Dipole moment | 0.0 D |
| Thermochemistry | |
| Specific heat capacity (C) |
224.64 J K−1 mol−1 |
| Std molar entropy (S |
328.57 J K−1 mol−1 |
| Std enthalpy of formation (ΔfH |
−225.2–−223.6 kJ mol−1 |
| Std enthalpy of combustion (ΔcH |
−4.825–−4.809 MJ mol−1 |
| Hazards | |
| GHS pictograms |    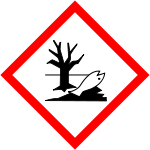 |
| GHS signal word | DANGER |
| H225, H304, H315, H336, H410 | |
| P210, P261, P273, P301+310, P331 | |
| EU Index | 601-008-00-2 |
| EU classification | |
| R-phrases | R11, R38, R50/53, R65, R67 |
| S-phrases | (S2), S16, S29, S33 |
| NFPA 704 | |
| Flash point | −4.0 °C (24.8 °F; 269.1 K) |
| 223.0 °C (433.4 °F; 496.1 K) | |
| Explosive limits | 1.05–6.7% |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 500 ppm (2000 mg/m3)[2] |
| REL (Recommended) |
TWA 85 ppm (350 mg/m3) C 440 ppm (1800 mg/m3) [15-minute][2] |
| IDLH (Immediate danger) |
750 ppm[2] |
| Related compounds | |
| Related alkanes |
|
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
n-Heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is a 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and isooctane which is expressed as the percentage of isooctane in heptane and is listed on pumps for gasoline dispensed in the United States and internationally.
Uses
Heptane (and its many isomers) is widely applied in laboratories as a totally non-polar solvent. As a liquid, it is ideal for transport and storage. In the grease spot test, heptane is used to dissolve the oil spot to show the previous presence of organic compounds on a stained paper. This is done by shaking the stained paper in a heptane solution for about half a minute.
Aqueous bromine may be distinguished from aqueous iodine by its appearance after extraction into heptane. In water, both bromine and iodine appear brown. However, iodine turns purple when dissolved in heptane, whereas the bromine solution remains brown.
Heptane is commercially available as mixed isomers for use in paints and coatings, as the rubber cement solvent "Bestine", the outdoor stove fuel "Powerfuel" by Primus, as pure n-Heptane for research and development and pharmaceutical manufacturing and as a minor component of gasoline.
Octane rating scale
n-Heptane is the zero point of the octane rating scale. It is undesirable in petrol, because it burns explosively, causing engine knocking, as opposed to branched-chain octane isomers, which burn more slowly and give better performance. It was chosen as the zero point of the scale because of the availability of very high purity n-heptane, unmixed with other isomers of heptane or other alkanes, distilled from the resin of Jeffrey Pine and from the fruit of Pittosporum resiniferum. Other sources of heptane and octane, produced from crude oil, contain a mixture of different isomers with greatly differing ratings and do not give as precise a zero point. ChevronPhillips Specialty Chemical produces a specialized grade of high purity n-Heptane (>99.0%) from crude oil through precision refining and distillation that is used in the measurement of octane rating for fuels.
Isomers and enantiomers
Heptane has nine isomers, or eleven if enantiomers are counted:
- Heptane (n-heptane), H3C–CH2–CH2–CH2–CH2–CH2–CH3,
- 2-Methylhexane (isoheptane), H3C–CH(CH3)–CH2–CH2–CH2–CH3,
- 3-Methylhexane, H3C–CH2–C*H(CH3)–CH2–CH2–CH3 (chiral),
- 2,2-Dimethylpentane, (H3C)3–C–CH2–CH2–CH3,
- 2,3-Dimethylpentane, (H3C)2–CH–C*H(CH3)–CH2–CH3 (chiral),
- 2,4-Dimethylpentane, (H3C)2–CH–CH2–CH–(CH3)2,
- 3,3-Dimethylpentane, H3C–CH2–C(CH3)2–CH2–CH3,
- 3-Ethylpentane, H3C–CH2–CH(CH2CH3)–CH2–CH3,
- 2,2,3-Trimethylbutane, CH3–C(CH3)2–CH(CH3)–CH3, this isomer is also known as pentamethylethane and triptane.[3]
References
- ↑ "n-heptane – Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 2 January 2012.
- ↑ 2.0 2.1 2.2 2.3 "NIOSH Pocket Guide to Chemical Hazards #0312". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Isomers. Members.optushome.com.au. Retrieved on 2012-03-04.
External links
- International Chemical Safety Card 0657 (n-heptane)
- International Chemical Safety Card 0658 (2-methylhexane)
- NIOSH Pocket Guide to Chemical Hazards
- Material Safety Data Sheet for Heptane
- Phytochemical database entry
| ||||||||||
