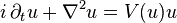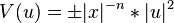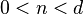Hartree equation
In 1927, a year after the publication of the Schrödinger equation, Hartree formulated what are now known as
the Hartree equations for atoms, using the concept of self-consistency that Lindsay had introduced in his study of many electron systems in the context of Bohr theory. Hartree assumed that the nucleus together with the electrons formed a spherically symmetric field. The charge distribution of each electron was the solution of the Schrödinger equation for an electron in a potential 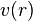 , derived from the field. Self-consistency required that the final field, computed from the solutions was self-consistent with the initial field and he called his method the self-consistent field method.
, derived from the field. Self-consistency required that the final field, computed from the solutions was self-consistent with the initial field and he called his method the self-consistent field method.
In order to solve the equation of an electron in a spherical potential, Hartree first introduced atomic units to eliminate physical constants. Then he converted the Laplacian from Cartesian to spherical coordinates to show that the solution was a product of a radial function 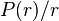 and a spherical harmonic with an angular quantum number
and a spherical harmonic with an angular quantum number 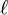 , namely
, namely 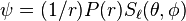 . The equation for the radial function was
. The equation for the radial function was
In mathematics, the Hartree equation, named after Douglas Hartree, is
in 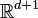 where
where
and
The non-linear Schrödinger equation is in some sense a limiting case.
![d^2P(r)/dr^2 + [2(E-v(r)) - \ell(\ell+1)/r^2]P(r)=0.](../I/m/eead302a713af05815f627165723ff4a.png)