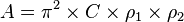Hamaker constant
The Hamaker constant A can be defined for a Van der Waals (VdW) body-body interaction:
where 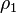 and
and 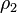 are the number of atoms per unit volume in two interacting bodies and C is the coefficient in the particle-particle pair interaction.[1]
are the number of atoms per unit volume in two interacting bodies and C is the coefficient in the particle-particle pair interaction.[1]
The Hamaker constant provides the means to determine the interaction parameter C from the Van der Waals pair potential, 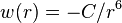 .
.
Hamaker's method and the associated Hamaker constant ignores the influence of an intervening medium between the two particles of interaction. In the 1950s Lifshitz developed a description of the VdW energy but with consideration of the dielectric properties of this intervening medium (often a continuous phase).
The Van der Waals forces are effective only up to several hundred angstroms. When the interactions are too far apart the dispersion potential decays faster than 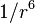 ; this is called the retarded regime and the result is a Casimir–Polder force.
; this is called the retarded regime and the result is a Casimir–Polder force.
See also
- Hamaker theory
- Van der Waals Forces
- Intermolecular forces
References
- ↑ Seung-woo Lee and Wolfgang M. Sigmund."AFM study of repulsive Van der Waals forces between Teflon AF thin film and silica or alumina." Colloids and Surfaces A: Physicochemical and Engineering Aspects. Volume 204, Issues 1-3, 23 May 2002, Pages 43–50
| ||||||||||||||||||||||||||