Hafnium tetraiodide
 | |
| Identifiers | |
|---|---|
| 13777-23-6 | |
| Properties | |
| HfI4 | |
| Molar mass | 686.11[1] |
| Appearance | red-orange[1] |
| Density | 5.60 g/cm3[1] |
| Melting point | 449 °C[1] |
| Boiling point | 394 °C (subl.)[1] |
| Structure | |
| Crystal structure | Monoclinic, mS40 |
| Space group | C2/c, No. 15[2] |
| Lattice constant | a = 1.1787 nm, b = 1.1801 nm, c = 1.2905 nm |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Hafnium tetraiodide is the inorganic compound with the formula HfI4. It is a red-orange, moisture sensitive, sublimable solid that is produced by heating a mixture of hafnium with excess iodine.[3] It is an intermediate in the crystal bar process for producing hafnium metal.
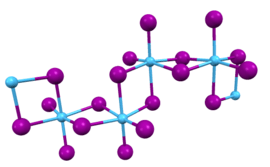
In this compound, the hafnium centers adopt octahedral coordination geometry. Like most binary metal halides, the compound is a polymeric. It is one-dimensional polymer consisting of chains of edge-shared bioctahedral Hf2I8 subunits, similar to the motif adopted by HfCl4. The nonbridging iodide ligands have shorter bonds to Hf than the bridging iodide ligands.[3]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.66. ISBN 1439855110.
- ↑ Krebs, B.; Sinram, D. (1980). "Hafniumtetrajodid HfI4: Struktur und eigenschaften. Ein neuer AB4-strukturtyp". Journal of the Less Common Metals 76: 7. doi:10.1016/0022-5088(80)90005-3.
- ↑ 3.0 3.1 Krebs, B.; Sinram, D. (1980). "Hafniumtetrajodid HfI4: Struktur und eigenschaften. Ein neuer AB4-strukturtyp". Journal of the Less Common Metals 76: 7. doi:10.1016/0022-5088(80)90005-3.
| ||||||||||